��Ŀ����
��2010?����ѧ�����������ߣ������ǵ�����ϢϢ��أ���ش����������е����⣺
��1��������������
��2�����ʵ��߲�ˮ���к��е�Ӫ������Ҫ��
��3��ȼ����Ҫ��������������ȼ����������������
��4����ɳ���Խ�˵���ƽ�����Ȼ�����ܹ���
��5��ˮ���ڱ��ϳ���ˮ������Ҫ�ɷ���̼��ƺ�������þ������������ʳ�׳�����������Ϊʳ���к��е����ᣨCH3COOH���������ԣ�����̼��ƺ�������þ��Ӧ����֪������̼��Ʒ�Ӧ�Ļ�ѧ����ʽΪ2CH3COOH+CaCO3=��CH3COO��2Ca+CO2��+2H2O����������������þ��Ӧ�Ļ�ѧ����ʽΪ��
��1��������������
̼��C��
̼��C��
Ԫ�أ���2�����ʵ��߲�ˮ���к��е�Ӫ������Ҫ��
����
����
��ˮ����3��ȼ����Ҫ��������������ȼ����������������
�ﵽȼ�����������¶ȣ����Ż�㣩
�ﵽȼ�����������¶ȣ����Ż�㣩
����4����ɳ���Խ�˵���ƽ�����Ȼ�����ܹ���
����
����
������ʡ����������ʽ���ڣ���5��ˮ���ڱ��ϳ���ˮ������Ҫ�ɷ���̼��ƺ�������þ������������ʳ�׳�����������Ϊʳ���к��е����ᣨCH3COOH���������ԣ�����̼��ƺ�������þ��Ӧ����֪������̼��Ʒ�Ӧ�Ļ�ѧ����ʽΪ2CH3COOH+CaCO3=��CH3COO��2Ca+CO2��+2H2O����������������þ��Ӧ�Ļ�ѧ����ʽΪ��
2CH3COOH+Mg��OH��2 =��CH3COO��2Mg+2H2O
2CH3COOH+Mg��OH��2 =��CH3COO��2Mg+2H2O
����������1�������л�������Ķ���شɣ�
��2�������߲�ˮ���ijɷֽ��з�����
��3������ȼ�յ��������ٿ�ȼ�������������۴ﵽȼ�����������¶ȼ��Ż�㣬����⣮
��4���˽���������Ȼ���еĴ���״̬��
��5�����ݸ��ֽⷴӦ���ص������кͷ�Ӧ�����κ�ˮ���з�����
��2�������߲�ˮ���ijɷֽ��з�����
��3������ȼ�յ��������ٿ�ȼ�������������۴ﵽȼ�����������¶ȼ��Ż�㣬����⣮
��4���˽���������Ȼ���еĴ���״̬��
��5�����ݸ��ֽⷴӦ���ص������кͷ�Ӧ�����κ�ˮ���з�����
����⣺��1������л�������Ļ�ѧԪ����Ҫ��̼Ԫ�أ������ܺ����⡢�����ȡ�������Ԫ�أ��ʴ�Ϊ��̼��C��
��2���߲�ˮ������Ҫ�ijɷ���ˮ������������ά���أ��ʴ�Ϊ��ά���أ�
��3����ȼ�յ�������ȼ����Ҫͬʱ���������������ٿ�ȼ�������������۴ﵽȼ�����������¶ȼ��Ż�㣬�ʴ�Ϊ���ﵽȼ�����������¶ȣ����Ż�㣩
��4�����ڽ�Ļ�ѧ���ʷdz��ȶ�������Ȼ������Ҫ���Ե�����ʽ���ڣ��ʴ�Ϊ������
��5��������������þ��Ӧ��������þ��ˮ���ʴ�Ϊ��2CH3COOH+Mg��OH��2 =��CH3COO��2Mg+2H2O
��2���߲�ˮ������Ҫ�ijɷ���ˮ������������ά���أ��ʴ�Ϊ��ά���أ�
��3����ȼ�յ�������ȼ����Ҫͬʱ���������������ٿ�ȼ�������������۴ﵽȼ�����������¶ȼ��Ż�㣬�ʴ�Ϊ���ﵽȼ�����������¶ȣ����Ż�㣩
��4�����ڽ�Ļ�ѧ���ʷdz��ȶ�������Ȼ������Ҫ���Ե�����ʽ���ڣ��ʴ�Ϊ������
��5��������������þ��Ӧ��������þ��ˮ���ʴ�Ϊ��2CH3COOH+Mg��OH��2 =��CH3COO��2Mg+2H2O
�����������Ҫ�����л���ĸ��ȼ�յ����������ೣ���Ļ�ѧ����֪ʶ�Լ���ѧ����ʽ����д������ֻ���������ܶ�����������ȷ���жϣ�

��ϰ��ϵ�д�
�����Ŀ
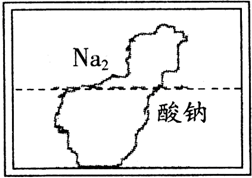 ��2010?����ʵ������һƿ��ɫ��Һ�����ڸ�ʴ�����ǩ������ͼ����
��2010?����ʵ������һƿ��ɫ��Һ�����ڸ�ʴ�����ǩ������ͼ����