��Ŀ����
(10��)��ȤС��ѡ�õ�15���е�Bװ��(װ������������)��CO2���������й�ʵ�顣
(1)ʵ������ȡCO2�Ļ�ѧ����ʽΪ______________________________��
(2)��ȤС����ʯ��ʯ����ε��������Ƶ����ᣬ������������ͨ�����ʯ��ˮ�У���ʯ��ˮʼ��û�л�������ʯ��ˮΪʲôû�л����أ��������ñ���ʵ����Ʒ����ѡ�Լ�(��ѡ�Լ���Na2CO3��Һ���ữ��AgNO3��Һ����ɫʯ����Һ)���̽�����̣�
��������롿��____________����____________�����������ֲ���ͬʱ���ڡ�
�������ʵ�顿�������ʵ�鷽������֤����ٺͲ���ڣ�����������������±���

(1)ʵ������ȡCO2�Ļ�ѧ����ʽΪ______________________________��
(2)��ȤС����ʯ��ʯ����ε��������Ƶ����ᣬ������������ͨ�����ʯ��ˮ�У���ʯ��ˮʼ��û�л�������ʯ��ˮΪʲôû�л����أ��������ñ���ʵ����Ʒ����ѡ�Լ�(��ѡ�Լ���Na2CO3��Һ���ữ��AgNO3��Һ����ɫʯ����Һ)���̽�����̣�
��������롿��____________����____________�����������ֲ���ͬʱ���ڡ�
�������ʵ�顿�������ʵ�鷽������֤����ٺͲ���ڣ�����������������±���

.(10��)(1)CaCO3+2HCl��CaCl2+H2O+CO2��(2��) (2)�����롿�ٳ���ʯ��ˮ�Ѿ�ʧЧ(����)(1��) ��CO2�л���HCl(1��) ����֤��
(˵����1.���������Ҫ��Ӧ��2.��֤�����ʱ�����·���Ҳ��ȷ��ȡԭ����ʯ��ˮ�μ�������ɫʯ����Һ����������ɫ����������ȷ��3.����������Ҳ����)

(˵����1.���������Ҫ��Ӧ��2.��֤�����ʱ�����·���Ҳ��ȷ��ȡԭ����ʯ��ˮ�μ�������ɫʯ����Һ����������ɫ����������ȷ��3.����������Ҳ����)
������1�����ݷ���ʽ����дע������ǣ���2��û�����˵�����ܱ��ʻ�����Ȼ����ٽ�һ�����ǣ���֤��Ҫ�ǿ�����Һ���Ƿ����������ƺ��Ȼ��⼴�ɣ�
��𣺽⣺��1����Ӧ����̼��ƺ��Ȼ��⣬���������Ȼ��ơ�ˮ��������̼���ù۲취��ƽ���ɣ�������̼��������������ţ�
��2��û�����˵�����ܱ��ʻ�����Ȼ��⣬��֤���������Ƿ���ʣ�����Һ���Ƿ����������ƣ����뺬̼������ӵĿ������Σ�����г�����˵��û���ʣ����û�г���˵���Ѿ����ʣ���֤����Ĵ��ڣ�������������Һ���а�ɫ����˵�������Ȼ��⣬�����У�
�ʴ�Ϊ����1��CaCO3+2HCl=CaCl2+H2O+CO2��
��2�������롿�ٳ���ʯ��ˮ�Ѿ�ʧЧ�����ʣ� ��CO2�л���HCl����֤��
��𣺽⣺��1����Ӧ����̼��ƺ��Ȼ��⣬���������Ȼ��ơ�ˮ��������̼���ù۲취��ƽ���ɣ�������̼��������������ţ�
��2��û�����˵�����ܱ��ʻ�����Ȼ��⣬��֤���������Ƿ���ʣ�����Һ���Ƿ����������ƣ����뺬̼������ӵĿ������Σ�����г�����˵��û���ʣ����û�г���˵���Ѿ����ʣ���֤����Ĵ��ڣ�������������Һ���а�ɫ����˵�������Ȼ��⣬�����У�
�ʴ�Ϊ����1��CaCO3+2HCl=CaCl2+H2O+CO2��
��2�������롿�ٳ���ʯ��ˮ�Ѿ�ʧЧ�����ʣ� ��CO2�л���HCl����֤��
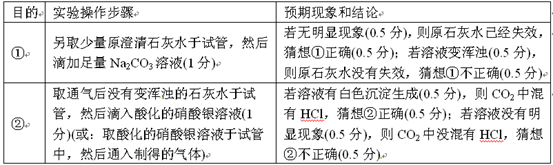
| Ŀ�� | ʵ��������� | Ԥ������ͽ��� |
| �� | ��ȡ����ԭ����ʯ��ˮ���Թܣ�Ȼ��μ�����Na2CO3��Һ | ��������������ԭʯ��ˮ�Ѿ�ʧЧ���������ȷ������Һ����ǣ���ԭʯ��ˮû��ʧЧ������ٲ���ȷ |
| �� | ȡͨ����û�б���ǵ�ʯ��ˮ���Թܣ�Ȼ������ữ����������Һ����ȡ�ữ����������Һ���Թ��У�Ȼ��ͨ���Ƶõ����壩 | ����Һ�а�ɫ�������ɣ���CO2�л���HCl���������ȷ������Һû������������CO2��û����HCl������ڲ���ȷ |

��ϰ��ϵ�д�
�����Ŀ