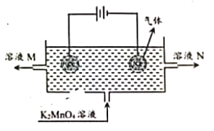
��Ŀ����
ͬѧ����ʵ���ҷ�����һƿû�����ӵ��������ƹ��壬Ϊ��̽��ƿ�й���ijɷ֣�ͬѧ��չ����̽����
��1��С��ͬѧȡ��14.6g��Ʒ������������ˮ����ܽ⣬�ٵ����������Ȼ�����Һ�����˵õ���ɫ����19.7g������ȡ��Ʒ���������Ƶ���������д��������̡�
��2����14.6g��Ʒ��������Ԫ�ص�����Ϊ ��
��3��С��ͬѧҲȡ14.6g��Ʒ������7.3%��ϡ����200g���۲쵽������Ϊ ����̼������ϡ����ķ�Ӧ��Ϊ�������У���һ����Na2CO3+HCl= NaHCO3+ NaCl �ڶ�����NaHCO3+ HCl= NaCl+H2O+CO2����ͼ����Ϊϡ���������������Ϊ����������������뻭ͼ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д����¹��ɺ��ܽ���ȫ��ȷ��һ����( )
A���Լ�������ʶ | B����ȫ����ʶ |
������Ӳˮ����ˮ���ӷ���ˮ����� ����������ά����ë��ά����ȼ������ζ ��������˿����˿���ô������� | �ٵ�ȼ��ȼ������ǰһ��Ҫ�鴿 ��ú���Ҫ����ͨ�硢�Ͻ��̻� �ۺ���ʳƷ������ȩ��Һ���� |
C�����������ʶ | D���Է������ʶ |
������ѹ�����������֮��ļ����С �ڻ���̿��ȥ��ζ������̿���������� �ۺ���ˮ���ã������ܽ�����¶����߶����� | �����г���֧����������� �ڲ˵��ú�ʱϴ������ �۱�������ɷ�ֹ����һ����ʴ |
A.A B.B C.C D.D


 ��ʾͬһ�¶�����NaCl��Һ�м������NaCl����Һ�����ʵ������仯
��ʾͬһ�¶�����NaCl��Һ�м������NaCl����Һ�����ʵ������仯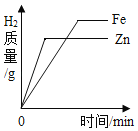 �ֱ�������������ۺ�п���м����������ͬ����������ϡ����
�ֱ�������������ۺ�п���м����������ͬ����������ϡ���� ��ʾˮ���ʵ������������������������
��ʾˮ���ʵ������������������������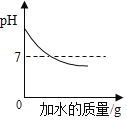 ��һ��������������������������Һ�в��ϼ�ˮ
��һ��������������������������Һ�в��ϼ�ˮ