��Ŀ����
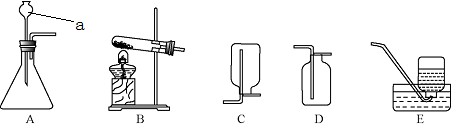
 ��ͼ��ʾ��ʵ������ȡCO2��װ��ͼ������ͼ�ش��������⣮
��ͼ��ʾ��ʵ������ȡCO2��װ��ͼ������ͼ�ش��������⣮��1��д������a��b�����ƣ�a��
����©��
����©��
��b����ƿ
��ƿ
����2��ʵ������ȡCO2����ҩƷ��
ʯ��ʯ
ʯ��ʯ
��ϡ����
ϡ����
���÷�Ӧ�Ļ�ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
����3����ʵ�����
�����ſ�����
�����ſ�����
���ռ�CO2�����ø÷��ռ���ԭ���ǣ�������̼���ܶȱȿ�����
������̼���ܶȱȿ�����
��4����μ���CO2�Ƿ��ռ�����
��ȼ�ŵ�ľ�����ü���ƿ�ڣ���ľ��Ϩ��˵���ռ���
��ȼ�ŵ�ľ�����ü���ƿ�ڣ���ľ��Ϩ��˵���ռ���
����5����μ����ռ���������CO2��
������ͨ��ʯ��ˮ����ʯ��ˮ����ǣ�˵���Ƕ�����̼
������ͨ��ʯ��ˮ����ʯ��ˮ����ǣ�˵���Ƕ�����̼
����6����CO2ʱ�����¼���������������ƿ��װ��ʯ��ʯ���ڼ��װ�������ԣ����ɷ�Һ©��������Һ�����ռ�����ȷ˳��Ϊ
�ڢ٢ۢ�
�ڢ٢ۢ�
����������1���������ճ����Ļ�ѧ�������ƺ���;��
��2��ʵ������ȡ������̼�ij���ҩƷ��ʯ��ʯ��ϡ���ᣬ��Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼��д����Ӧ�ķ���ʽ��
��3��������̼���ܶȱȿ�������������ˮ��ѡ����ʵ��ռ�������
��4������CO2�����Ƿ��ѳ�������ƿ�ķ����ǣ���ȼ�ŵ�ľ�����ü���ƿ�ڣ���ľ���Ƿ�Ϩ��
��5�����������̼��ʯ��ˮ����ʯ��ˮ�Ƿ����ǣ�
��6���÷���ȡ������̼�IJ����ǣ����װ�õ������ԡ�װҩƷ���ռ���
��2��ʵ������ȡ������̼�ij���ҩƷ��ʯ��ʯ��ϡ���ᣬ��Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼��д����Ӧ�ķ���ʽ��
��3��������̼���ܶȱȿ�������������ˮ��ѡ����ʵ��ռ�������
��4������CO2�����Ƿ��ѳ�������ƿ�ķ����ǣ���ȼ�ŵ�ľ�����ü���ƿ�ڣ���ľ���Ƿ�Ϩ��
��5�����������̼��ʯ��ˮ����ʯ��ˮ�Ƿ����ǣ�
��6���÷���ȡ������̼�IJ����ǣ����װ�õ������ԡ�װҩƷ���ռ���
����⣺��1����dz������������ƺ���;��a�dz���©����b����ƿ��
��2��ʵ������ȡ������̼�ij���ҩƷ��ʯ��ʯ��ϡ���ᣬ���е�̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�ķ���ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
��3����Ϊ������̼������ˮ���ܶȱȿ�����ֻ�ܲ��������ſ������ռ���
��4������CO2�����Ƿ��ѳ�������ƿ�ķ����ǣ���ȼ�ŵ�ľ�����ü���ƿ�ڣ���ľ��Ϩ��˵���ռ�����
��5�����������̼�ķ����ǣ�������ͨ��ʯ��ˮ����ʯ��ˮ����ǣ�˵���Ƕ�����̼����Ӧ�ķ���ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
��6���÷���ȡ������̼�IJ����ǣ����װ�õ������ԡ�װҩƷ���ȹ̺�Һ�����ռ�������̼����˳��Ϊ���ڢ٢ۢܣ�
�ʴ�Ϊ����4����ȼ�ŵ�ľ�����ü���ƿ�ڣ���ľ��Ϩ��˵���ռ�����
��5��������ͨ��ʯ��ˮ����ʯ��ˮ����ǣ�˵���Ƕ�����̼��
��6���ڢ٢ۢܣ�
��2��ʵ������ȡ������̼�ij���ҩƷ��ʯ��ʯ��ϡ���ᣬ���е�̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�ķ���ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
��3����Ϊ������̼������ˮ���ܶȱȿ�����ֻ�ܲ��������ſ������ռ���
��4������CO2�����Ƿ��ѳ�������ƿ�ķ����ǣ���ȼ�ŵ�ľ�����ü���ƿ�ڣ���ľ��Ϩ��˵���ռ�����
��5�����������̼�ķ����ǣ�������ͨ��ʯ��ˮ����ʯ��ˮ����ǣ�˵���Ƕ�����̼����Ӧ�ķ���ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
��6���÷���ȡ������̼�IJ����ǣ����װ�õ������ԡ�װҩƷ���ȹ̺�Һ�����ռ�������̼����˳��Ϊ���ڢ٢ۢܣ�
�ʴ�Ϊ����4����ȼ�ŵ�ľ�����ü���ƿ�ڣ���ľ��Ϩ��˵���ռ�����
��5��������ͨ��ʯ��ˮ����ʯ��ˮ����ǣ�˵���Ƕ�����̼��
��6���ڢ٢ۢܣ�
���������⿼����ʵ���ҳ����������ȡԭ������д������װ�á��ռ�װ�õ�ѡ���Լ���������������ȣ��ۺ��ԱȽ�ǿ���ؼ�����ȷ����װ�á��ռ�����ѡ������ݼ���ص�֪ʶ���ܹ�����ѧ����֪ʶǨ��������������Ҫ�ص����յ�֪ʶ��Ҳ���п����������ͣ�

��ϰ��ϵ�д�
�����Ŀ


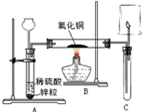 ��ͼ��ʾ��ʵ������ȡ��������������ԭ����ͭ��װ�ã�����ͼ�ش��������⣺
��ͼ��ʾ��ʵ������ȡ��������������ԭ����ͭ��װ�ã�����ͼ�ش��������⣺