��Ŀ����
����Ŀ��С��ͬѧȡij��ʯ��ʯ��Ʒ12.5g���вⶨʵ�飬�ֽ�100gϡ�������μ���ʯ��ʯ��Ʒ�У����ʲ�����ˮҲ�����뷴Ӧ������ַ�Ӧ����������������������±���ʾ��
��1�� | ��2�� | ��3�� | ��4�� | ��5�� | |
����ϡ���������/g | 20 | 20 | 20 | 20 | 20 |
���������������/g | 1.1 | 2.2 | m | 4.4 | 4.4 |
����㣺
(1)m��ֵ��
(2)ʯ��ʯ��Ʒ��̼��Ƶ�����������
(3)����ϡ�������ʵ����������Ƕ��٣���Ҫ��д��������̣�
���𰸡�(1)3.3��(2)80% ��(3) 9.125%
���������⣺(1)����μ��������ǰ���Σ�ÿ�ζ�����1.1g������̼�����ġ��嶼����4.4g������̼��˵��������û����ȫ��Ӧ��Ҳ����1.1g������mֵ��3.3��(2)����Ʒ��̼��Ƶ�����Ϊx���μӷ�Ӧ�Ȼ��������Ϊy��������Ŀ���ṩ����Ϣ��֪��̼��������ᷴӦ��ȫʱ�����ɵĶ�����̼������Ϊ4.4g��
CaCO3+2HCl=CaCl2+CO2��+H2O
100 73 44
x y 4.4g
![]() ��x=10g
��x=10g
![]() ��y=7.3g
��y=7.3g
ʯ��ʯ��Ʒ��̼��Ƶ����������� ![]() 100%=80%��
100%=80%��
����ϡ�������ʵ���������: ![]() 100%= 9.125%��
100%= 9.125%��
��(1) mֵ��3.3��(2)ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ80%��(3)����ϡ�������ʵ���������Ϊ9.125%��

����Ŀ����ѧ�������Դ�����ϡ�����ϢϢ�������������ѧ��ѧ֪ʶ�ش��й����⣺
(1)�Ž��з�����Ԫ�������ɲ����Ƴ�______________��ʹ��ѧѧϰ���о�����й��ɿ�ѭ��
(2)��ʯȼ���ѵõ��㷺���ɺ�ʹ�á���ʯȼ�ϰ���ú��___________����Ȼ����
(3)ijʳƷ��װ���ϵ�˵�����£�
��Ʒ���� | �������� |
���� | �ʼ�������ɰ�ǡ�С��ۡ�ֲ���͡����͡�ʳ�Ρ��㾫�����ɼ� |
��� | 400g |
���ط��� | ��������ֱ�䣬�����������ˬ�ĵط� |
������ | 18���� |
�������� | 2018��3��28�� |
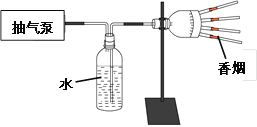
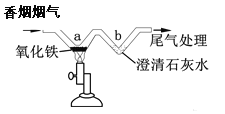
ijͬѧ�������ֱ�����Ϊ��ͣ�����Ӫ������ԭ��������Ϊ����Ҫ��ʳ______________�Բ��������û�е�Ӫ���ء�
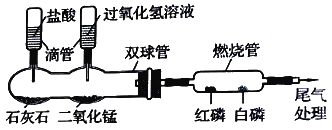
(4)�ڻ�ҩ���ҹ��Ŵ����Ĵ���֮һ���ڻ�ҩ��ըʱ�����ķ�ӦΪ��S + 2KNO3 + 3C=X + 3 CO2�� + N2������X�Ļ�ѧʽΪ__________��
(5)�ҹ��ĸ��������������磬�����ֹ���������������ڸ����·����û���Ӧʵ���캸�ӣ��÷�Ӧ�Ļ�ѧ����ʽΪ___________________________________________________��