��Ŀ����
27��ʵ������A��B��C��D��ƿ��ɫ��Һ���ֱ���ϡ���ᡢ����ʯ��ˮ������������Һ��̼������Һ���������е�һ�֣��ɹ�ʹ�õ�ʵ����Ʒ�У�pH��ֽ��ϡ���ᡢ���������Թܡ������ܣ�
��1���������ṩ��ʵ����Ʒ��������������ʣ������ʵ�鱨�棺
��������Ӧ���룺��
��
��2��С����Ϊ�������κ��Լ���ֻҪ��֧�Թܼ�����ɼ���ʵ�飮������ƿ�Լ����±�ţ�����������Һ������ϣ�����ʵ������¼���£�

��С�����Եõ��Ľ���Ϊ��
��1���������ṩ��ʵ����Ʒ��������������ʣ������ʵ�鱨�棺

��������Ӧ���룺��
ϡ���ᣨ��H2SO4��
��A��Һ�����ݲ���������������
��
C��Һ�г���������D�����𰸺�������
����2��С����Ϊ�������κ��Լ���ֻҪ��֧�Թܼ�����ɼ���ʵ�飮������ƿ�Լ����±�ţ�����������Һ������ϣ�����ʵ������¼���£�

��С�����Եõ��Ľ���Ϊ��
A�DZ���ʯ��ˮ[��Ca��OH��2]��B��̼���ƣ�C��ϡ���ᣬD���������ƣ���NaOH��
����������1���������������ʵIJ�ͬ���ʺ�ʵ�����ƶ�ʵ������
��2����������������֮��ķ�Ӧ��������ƶϼ��ɣ�
��2����������������֮��ķ�Ӧ��������ƶϼ��ɣ�
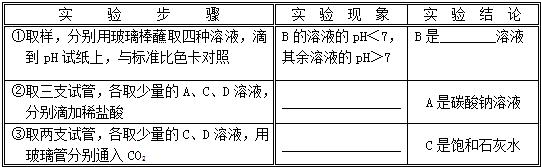
����⣺��1������Ϊ�����������У�ֻ��ϡ���������ᣬ���ph��7���ʿ��ж�BΪϡ������Һ��
�����AΪ̼������Һ�����ϡ���ᷴӦ����������������������
����Ϊ������̼������ʹ����ʯ��ˮ����ǣ������C�DZ���ʯ��ˮ��Һ�����г���������D������
�ʴ�Ϊ��ϡ���ᣨ��H2SO4����A��Һ�����ݲ���������������C��Һ�г���������D�����𰸺������ɣ�
��2����Ϊ̼�������������Ʒ�Ӧ����̼��Ƴ�������ϡ���ᷴӦ���ɶ�����̼���壬��BΪ̼������Һ��AΪ�������ƣ�����ʯ��ˮ����CΪϡ���ᣬ��DΪ����������Һ��
��ѡAΪ�������ƣ�����ʯ��ˮ����BΪ̼������Һ��CΪϡ���ᣬDΪ����������Һ��
�����AΪ̼������Һ�����ϡ���ᷴӦ����������������������
����Ϊ������̼������ʹ����ʯ��ˮ����ǣ������C�DZ���ʯ��ˮ��Һ�����г���������D������
�ʴ�Ϊ��ϡ���ᣨ��H2SO4����A��Һ�����ݲ���������������C��Һ�г���������D�����𰸺������ɣ�
��2����Ϊ̼�������������Ʒ�Ӧ����̼��Ƴ�������ϡ���ᷴӦ���ɶ�����̼���壬��BΪ̼������Һ��AΪ�������ƣ�����ʯ��ˮ����CΪϡ���ᣬ��DΪ����������Һ��
��ѡAΪ�������ƣ�����ʯ��ˮ����BΪ̼������Һ��CΪϡ���ᣬDΪ����������Һ��
������������Ҫ�ǿ���ͬѧ�ǵ��ۺϷ�������������Ҫ��ͬѧ�Ǿ߱��йػ�����Ļ���֪ʶ������Ҫ��ʵ������ľ����ͷ����������ѧʵ�����������������ʱ��Ҫ���������Ŀ��������������ϵʵ�ʣ���һ�����ƶϣ�

��ϰ��ϵ�д�
�����Ŀ

 ��2006?��������ģ��ʵ������A��B��C��D��E��ƿʧȥ��ǩ����Һ����֪���Ƿֱ���̼���ơ��Ȼ���������ơ�ϡ������������е�ijһ����Һ����ͼΪ����ʱ������Һ������ϵIJ���ʵ���������С�������ʾ���������ɣ���������ʾ�г������ɣ���-����ʾ��������������������ɣ�����ʵ�����ش��������⣺
��2006?��������ģ��ʵ������A��B��C��D��E��ƿʧȥ��ǩ����Һ����֪���Ƿֱ���̼���ơ��Ȼ���������ơ�ϡ������������е�ijһ����Һ����ͼΪ����ʱ������Һ������ϵIJ���ʵ���������С�������ʾ���������ɣ���������ʾ�г������ɣ���-����ʾ��������������������ɣ�����ʵ�����ش��������⣺