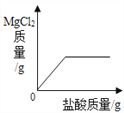
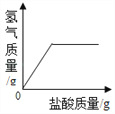
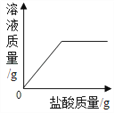
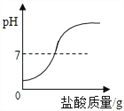
��Ŀ����
����Ŀ����8�֣�̼Ԫ��������������ʵĻ���Ԫ�ء�
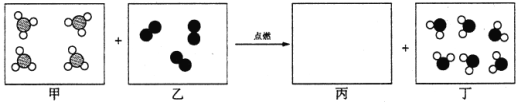
��1�����к�̼Ԫ�ص������У������л������ ������ĸ��ţ���
A��̼��� B���Ҵ���C2H5OH �� C��������̼
��2����ʯȼ����Ҫ����ú�� ����Ȼ�������Ƕ�����̼Ԫ�أ�������Ȼ������Ҫ�ɷ�
�� ��д��ѧʽ����
��3���ܶ���Ȼ��ʯ�к���̼Ԫ�أ����̿����Ҫ�ɷ���̼���̣�MnCO3����������Ԫ�صĻ��ϼ� ��
��4���� 440����ѹ�����£��������������̼��Ӧ�����ɽ��ʯ��C����̼���ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5����̼���ࡱ����֪����Ĺ�����ϣ�����ͼ��ʾ������̼Ԫ����ɣ����ж�ṹ�����Ժá�����ʯ���к�ǿ����������������ˮ�����������ʯ�ͼ������Կɻָ�ԭ״�����й���̼�����˵����ȷ���� ������ĸ��ţ���

A������������ B�����ظ�ʹ�� C���ɴ�������ʯ��й©
���𰸡���1��B ��2�� ʯ�� CH4 ��3�� +2
��4��4Na+3CO2![]() C+2Na2CO3 ��5��ABC
C+2Na2CO3 ��5��ABC
��������
�����������1���л����Ǻ�̼Ԫ�صĻ������������̼��̼��ơ�һ����̼��̼��ȣ���Ȼ��̼Ԫ�أ�����Ȼ���������ѡB
��2����ʯȼ����Ҫ����ú��ʯ�͡���Ȼ����������Ȼ������Ҫ�ɷ��Ǽ��飬��ѧʽΪ��CH4
��3������Ԫ�ػ��ϼ۵�һ����ɣ��ڻ������У��������ϼ۵Ĵ�����Ϊ0����CO3�Ļ��ϼ�Ϊ-2������Ԫ�صĻ��ϼ�Ϊ+2
��4���� 440����ѹ�����£��������������̼��Ӧ�����ɽ��ʯ��C����̼���ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ��4Na+3CO2![]() C+2Na2CO3
C+2Na2CO3
��5����̼���ࡱ����֪����Ĺ�����ϣ���̼Ԫ����ɣ����ж�ṹ�����Ժá�����ʯ���к�ǿ����������������ˮ�����������ʯ�ͼ������� �ɻָ�ԭ״����A�����������ԣ�B�����ظ�ʹ�ã�C���ɴ�������ʯ��й©����ȷ

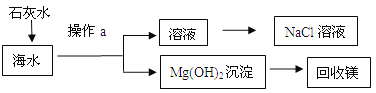
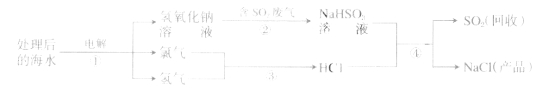
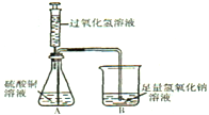
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�