��Ŀ����
�����ǵ���Ҫ�ɷ���̼��ƣ�Ϊ�ⶨ������̼��Ƶ�����������С����С��ͬѧ����������ʵ�飮��ش�������⣺
��1��ʵ����̺Ͳⶨ�����ʵ������������ʾ������ʾ�������������ᷴӦ��
�ٳ�ַ�Ӧ��С�����ݡ���Ӧ�����м��ٵ�������������ɵ�CO2�����ʵ�����________mol��Ȼ�����CO2������õ�����̼��Ƶ���������������д��С���ļ�����̺ͽ��������ȷ��0.1%����ͬ��
��С�ø��ݡ����Dz�������Ϊ4g������õ�����̼��Ƶ���������Ϊ________��
��2��С����С����õĵ�����̼��Ƶ����������в������������п��ܵ�ԭ��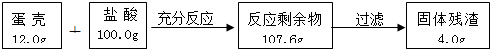
�⣺��1���ٳ�ַ�Ӧ��С�����ݡ���Ӧ�����м��ٵ�������������ɵ�CO2��������12.0g+100.0g-107.6g=4.4g��CO2�����ʵ���=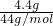 =0.1mol
=0.1mol
��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
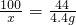
x=10.0g
��̼��Ƶ���������=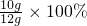 =83.3%
=83.3%
��̼��Ƶ���������83.3%��
�ڵ�����̼��Ƶ���������= =66.7%��
=66.7%��
��2��С���������õĹ����������δ���ʵ�ʹ����������С��4g����С�ü������õ����ݡ�4.4g���а����˷�Ӧ����������ӷ����Ȼ����������������ʵ�����ɵĶ�����̼��������С��4.4g��������С����С����õĵ�����̼��Ƶ����������в��
�ʴ�Ϊ����1����0.1mol��83.3%����66.7%����2��С���������õĹ����������δ���ʵ�ʹ����������С��4g����С�ü������õ����ݡ�4.4g���а����˷�Ӧ����������ӷ����Ȼ����������������ʵ�����ɵĶ�����̼��������С��4.4g����
��������1�����ݼ����ǵ���Ҫ�ɷ���̼��ƣ����ᷴӦ�Dz�������Ϊ4.3g����̼��������ᷴӦ����ɼ���̼��Ƶ��������ټ��㵰����̼��Ƶ�����������
Ҳ�����������غ㶨�ɣ����÷�Ӧǰ����������������������������̼�����������û�ѧ��Ӧ����ʽ������̼��Ƶ���������һ�����㵰����̼��Ƶ�����������
��2������ʵ���й��������ij�����������ռ��ȷ�������������
���������⿼��ѧ�����û�ѧ��Ӧ����ʽ�ļ��㣬��ȷ��Ӧ����������������������غ������������̼�������ǽ��Ĺؼ���
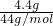 =0.1mol
=0.1mol��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
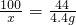
x=10.0g
��̼��Ƶ���������=
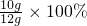 =83.3%
=83.3%��̼��Ƶ���������83.3%��
�ڵ�����̼��Ƶ���������=
 =66.7%��
=66.7%����2��С���������õĹ����������δ���ʵ�ʹ����������С��4g����С�ü������õ����ݡ�4.4g���а����˷�Ӧ����������ӷ����Ȼ����������������ʵ�����ɵĶ�����̼��������С��4.4g��������С����С����õĵ�����̼��Ƶ����������в��
�ʴ�Ϊ����1����0.1mol��83.3%����66.7%����2��С���������õĹ����������δ���ʵ�ʹ����������С��4g����С�ü������õ����ݡ�4.4g���а����˷�Ӧ����������ӷ����Ȼ����������������ʵ�����ɵĶ�����̼��������С��4.4g����
��������1�����ݼ����ǵ���Ҫ�ɷ���̼��ƣ����ᷴӦ�Dz�������Ϊ4.3g����̼��������ᷴӦ����ɼ���̼��Ƶ��������ټ��㵰����̼��Ƶ�����������
Ҳ�����������غ㶨�ɣ����÷�Ӧǰ����������������������������̼�����������û�ѧ��Ӧ����ʽ������̼��Ƶ���������һ�����㵰����̼��Ƶ�����������
��2������ʵ���й��������ij�����������ռ��ȷ�������������
���������⿼��ѧ�����û�ѧ��Ӧ����ʽ�ļ��㣬��ȷ��Ӧ����������������������غ������������̼�������ǽ��Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ