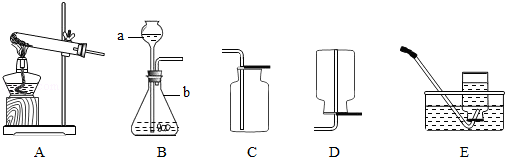��Ŀ����
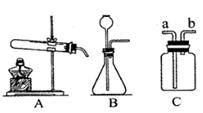
����ʵ��װ�ÿɽ��������Ʊ����ռ�������ʵ�顣��ش��й����⣺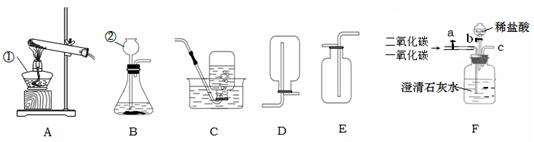
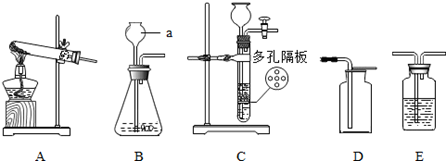
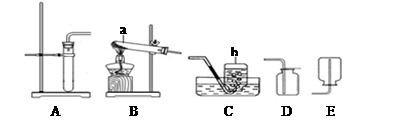
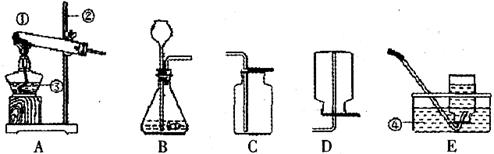
��1�������ٵ������� �������ڵ������� ��
��2�����������������̻����ȡ������ѡ��ķ���װ���� �����ţ�����Ӧ�Ļ�ѧ����ʽΪ ��
��3��ͨ��װ��F������Һ��ҩƷ�����������Խ�������̼��һ����̼�Ļ��������з��롣���ȴ���a�����ƿ�й۲쵽�������� ����ʱ�ӵ���c�ݳ���������Ҫ�� ��һ��ʱ��رջ���a������b���μ�������ϡ���ᣬ��ʱ�ݳ���������Ҫ�� ��������Ӧ�Ļ�ѧ����ʽΪ ��
���������������1�������ٵ������ǣ��ƾ��ƣ������ڵ�����������Һ��ҩƷ����2�����������������̻����ȡ��������Ϊ�ǹ��������ȡ���壬��ѡ��ķ���װ����A, ��Ӧ�Ļ�ѧ����ʽΪ: 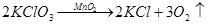 ;��3��ͨ��װ��F������Һ��ҩƷ�����������Խ�������̼��һ����̼�Ļ��������з��롣���ݶ�����̼��һ����̼��������֪�����ȴ���a�����ƿ�й۲쵽�������dz���ʯ��ˮ����ǣ�������ɫ��������ʱ�ӵ���c�ݳ���������Ҫ��һ����̼��һ��ʱ��رջ���a������b���μ�������ϡ���ᣬ��ʱ�ݳ���������Ҫ�Ƕ�����̼��������Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl��CaCl2+CO2��+H2O
;��3��ͨ��װ��F������Һ��ҩƷ�����������Խ�������̼��һ����̼�Ļ��������з��롣���ݶ�����̼��һ����̼��������֪�����ȴ���a�����ƿ�й۲쵽�������dz���ʯ��ˮ����ǣ�������ɫ��������ʱ�ӵ���c�ݳ���������Ҫ��һ����̼��һ��ʱ��رջ���a������b���μ�������ϡ���ᣬ��ʱ�ݳ���������Ҫ�Ƕ�����̼��������Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl��CaCl2+CO2��+H2O
���㣺�������ȡ˼·�ͷ�����������̼������