��Ŀ����
[��������]ʵ��С���ͬѧ���֣�����ͭ��Ũ����Ļ���������һ����ɫ�д̼�����ζ���������ɫ��Һ��[��������]��ijЩ�����õĽ�������ͭ������һЩǿ�����Ե��ᣨ��Ũ���ᣩ������Ӧ������ˮ��������������ͬʱ���ɶ��������ͭ�Σ�
������ͭ����ˮ����Һ�����ԣ�
[��������]��1���������ɵ���ɫ��Һ��С����Ϊ������Cu��NO3��2��Һ��С���Ϊ��������Cu��NO3��2��Һ��������������ͭ��Һ��С骵�������______��
��2��д��ͭ��Ũ���Ṳ�ȵĻ�ѧ����ʽ______ CuSO4+2H2O+SO2��
���𰸡�������[��������]�����ݽ������˳�����������Ľ��������ᷴӦ�����������ָϡ��������ᣬ������Ũ��������ᣬ��ΪŨ�����������ǿ�����ԣ���1��������������Ϣ��ͭ�ܺ�Ũ���ᷴӦ��������ͭ�Ͷ���������ˮ����2�������ķ�Ӧ���������غ㶨�ɣ�Ԫ�ص�����䣻��3�����ɵĶ����������ж����壬����Ⱦ������
[ʵ����֤]����1��������Һ��ij�����ӵ�ʱ��Ҫ�ų�����������ɵĸ��ţ�����ó�������ۣ���2����Ӧ���ٶȺͷ�Ӧ���Ũ���йأ�
����⣺[֪ʶ�ع�]���ݽ������˳�����������Ľ��������ᷴӦ������ͭ����������ϡ���ᡢ���ᷴӦ����������
��1��ͭ��Ũ���ᷴӦ������������ͭ����Ϊ��Ӧǰ��Ԫ�ص�����䣮
��2��дͭ��Ũ���Ṳ�ȵĻ�ѧ����ʽʱҪ��д����Ӧ���������Ļ�ѧʽ����ƽ����ע��������ţ�
��3����Ϊ�����������ж���������������Բ������������Ӧ������ȡ����ͭ��
[ʵ����֤]����������ͭ��Һ���Ƿ�������ʱ�����ܼ�����������ӣ���Ϊ����ͭ��Ҳ���������
��1��������ʯ����Һ����Ϊ����ͭҲ�����ԣ�
��2��������ͭ��������Ũ�����������м��ȣ���Ӧһ��ʱ���Ӧ������������Ϊ���ŷ�Ӧ�Ľ��У�Ũ���������������С��ϡ������ͭ����Ӧ��
�ʴ𰸣�����
��1����Ӧǰ��Ԫ������䣨�����÷֣�
��2��Cu+2H2SO4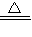 CuSO4+2H2O+SO2��
CuSO4+2H2O+SO2��
��3�����ɵĶ����������Ⱦ�����������𰸾����֣�
[ʵ����֤]��ֻ��˵������SO42-������ȷ��������ʣ�ࣨ�����𰸾����֣�
��1������ͭ��ҺҲ�����Ե�
��2�����ŷ�Ӧ�Ľ��У�Ũ���������������С��ϡ������ͭ����Ӧ
�������������г�������ǰ��ѧ֪ʶ���ɳ�ͻʱ������Ҫ�ı�˼ά���ƣ����ڽ�����֪ʶ����Ϊ��ǰ��ѧ��ijЩ֪ʶ��һ���ľ����ԣ�
[ʵ����֤]����1��������Һ��ij�����ӵ�ʱ��Ҫ�ų�����������ɵĸ��ţ�����ó�������ۣ���2����Ӧ���ٶȺͷ�Ӧ���Ũ���йأ�
����⣺[֪ʶ�ع�]���ݽ������˳�����������Ľ��������ᷴӦ������ͭ����������ϡ���ᡢ���ᷴӦ����������
��1��ͭ��Ũ���ᷴӦ������������ͭ����Ϊ��Ӧǰ��Ԫ�ص�����䣮
��2��дͭ��Ũ���Ṳ�ȵĻ�ѧ����ʽʱҪ��д����Ӧ���������Ļ�ѧʽ����ƽ����ע��������ţ�
��3����Ϊ�����������ж���������������Բ������������Ӧ������ȡ����ͭ��
[ʵ����֤]����������ͭ��Һ���Ƿ�������ʱ�����ܼ�����������ӣ���Ϊ����ͭ��Ҳ���������
��1��������ʯ����Һ����Ϊ����ͭҲ�����ԣ�
��2��������ͭ��������Ũ�����������м��ȣ���Ӧһ��ʱ���Ӧ������������Ϊ���ŷ�Ӧ�Ľ��У�Ũ���������������С��ϡ������ͭ����Ӧ��
�ʴ𰸣�����
��1����Ӧǰ��Ԫ������䣨�����÷֣�
��2��Cu+2H2SO4
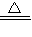 CuSO4+2H2O+SO2��
CuSO4+2H2O+SO2����3�����ɵĶ����������Ⱦ�����������𰸾����֣�
[ʵ����֤]��ֻ��˵������SO42-������ȷ��������ʣ�ࣨ�����𰸾����֣�
��1������ͭ��ҺҲ�����Ե�
��2�����ŷ�Ӧ�Ľ��У�Ũ���������������С��ϡ������ͭ����Ӧ
�������������г�������ǰ��ѧ֪ʶ���ɳ�ͻʱ������Ҫ�ı�˼ά���ƣ����ڽ�����֪ʶ����Ϊ��ǰ��ѧ��ijЩ֪ʶ��һ���ľ����ԣ�

��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
�����Ŀ