��Ŀ����
����Ŀ��ijУ�о���ѧϰС�������һ����Ȥ��ʵ��̽��:
[�������]ʵ������һƿ���õ�NaOH�����ʳ̶�����?
[��Ʒ���]�ȳ�ȡ21.2g��NaOH��Ʒ(����ΪNa2CO3),�����Һ��Ȼ������Һ����μ���һ������������ϡ����ֱ������,��������CO2�����������Na2CO3���������Ӷ���һ��ȷ����Ʒ��NaOH������������
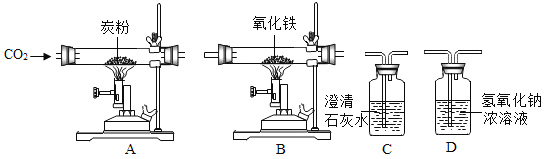
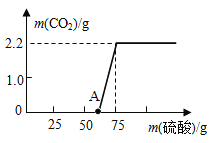
[����ʵ��]ʵ���ü���ϡ��������������CO2�����������ϵ����ͼ��ʾ��
[���ݴ���]д�����¼������:
��1������Ʒ��Na2CO3������Ϊ����?
��2������Ʒ��NaOH����������Ϊ����?
��3��NaOH���ʵ�ԭ��(�û�ѧ����ʽ��ʾ)
���𰸡���1��5.3g����2��75.0% ��3��2NaOH+CO2=Na2CO3+H2O
��������
������ͼ������й������̽������Ŀѡ�����������̼��Ӧ�ļ��������������Ƶı��ʳ̶ȣ����ܼ�����Ʒ���������������������������ؼ��ǿ�����ͼ��ĺ��壬ע��ͼ��������ĵ�����壬Ȼ������Ŀ���������������⡣
��ͼ���֪���ڼ�����Ʒ�е�����ʱ����ϡ���ᣬ��������̼���������ԭ�����ڼ�����Һ�У����������кͷ�Ӧ�����������壬����������������Ϊ50���ʱ����ʼ����Ʒ�е�̼���Ʒ�����Ӧ�����ɶ�����̼���壬�����ŷ�Ӧ�Ľ��У��������μ�����ʱ������������Ϊ2.2g�����Ҳ��ٱ仯��˵����Ʒ�е�̼������ȫ��Ӧ��ϣ��ɴ˿ɸ��ݶ�����̼�������������Ʒ��̼���Ƶ�������ͬ��Ҳ�ɼ����ԭ��Ʒ���������Ƶ��������Ӷ��ó���Ʒ���������Ƶ������������������£�
[���ݴ���]��ͼ�е�֪������CO2�������Ϊ2.2g��
��1���⣺�����Ʒ��Na2CO3������Ϊx����
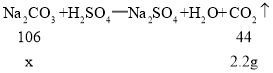
![]()
x=5.3g
��2������Ʒ��NaOH��������Ϊ��![]()
[��˼�뽻��]��ͼ���֪��ʼ��������ʱ���������ɣ���һ�������������������Ʒ�Ӧ�����������Ʒ�Ӧ�������̼���Ʒ�Ӧ���ɶ�����̼���������Ʊ������������ƺͶ�����̼��Ӧ����̼���ƺ�ˮ����Ӧ����ʽΪ��2NaOH+CO2=Na2CO3+H2O��

 �Ķ��쳵ϵ�д�
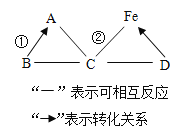
�Ķ��쳵ϵ�д�����Ŀ���ס��ҡ����������ʵ�ת����ϵ��������ͼ��ʾ����������ʾ��Ӧ����һ��ʵ�֣��������ʺͷ�Ӧ������ʡ�ԣ�������ѡ���в��ܰ�ͼʾת����ϵʵ�ֵ��ǣ�������
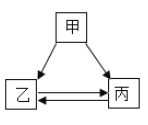
ѡ�� | �� | �� | �� |
A | H2SO4 | H2 | H2O |
B | NaOH | NaCl | NaNO3 |
C | Na2O | NaOH | Na2CO3 |
D | Ca��OH��2 | CaCl2 | CaCO3 |
A. AB. BC. CD. D