��Ŀ����
Ϊ�ⶨijNaCl��Na2CO3�����������ɣ�С��ͬѧȡ16 g�û��������ձ��У�����μ���ϡ���ᣨÿ�μ���ϡ���������Ϊ25g��������Ӧ��ȫ�õ������������ϵ��| ����ϡ����Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� |
| �ձ�����Ӧ�������������/g | 122.2 | 146.1 | 170.0 | 193.9 | 218.9 |
��1��ԭ����������Na2CO3��������
��2��������ϡ��������������ǡ����ȫ��Ӧʱ��������Һ��������������������������ȷ��0.1��
���𰸡���������1��������������֪��ǰ�Ĵ�ʵ�飬ÿ�μ�25gϡ���ᣬ������������1.1g�������εμ�ϡ�����������ޱ仯��˵�����Ĵ����������Na2CO3ǡ����ȫ��Ӧ����Ӧ�й�����CO24.4g�����ݻ�ѧ����ʽ�����ɼ�������뷴Ӧ��Na2CO3��������
��2�����ݻ�ѧ����ʽ����������ɵ��Ȼ��Ƶ�������Ȼ�������ȫ��Ӧ��������Һ����=����������+���뷴Ӧ�����������-����������������Һ����������=��������Ȼ��Ƶ�����+���ɵ��Ȼ��Ƶ�������Ȼ�������������������ʽ���㼴�ɣ�
����⣺��Ӧ�й�����CO2������Ϊ����122.2g+25g-146.1g��×4=4.4g��
����뷴Ӧ��Na2CO3������Ϊx��
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 117 44
x y 4.4g
�� ��
��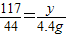
��֮�ã�x=10.6g��y=11.7g��
�������Na2CO3����Ϊ10.6g������NaCl������Ϊ11.7g��
����ȫ��Ӧ��������Һ����Ϊ��25g×4+16g-4.4g=111.6g��
����ȫ��Ӧ����Һ����������Ϊ��16g-10.6g+11.7g=17.1g��
����ȫ��Ӧ����Һ��������������Ϊ�� ��15.3%��
��15.3%��
��ԭ������Na2CO3������Ϊ10.6g����Ӧ��������Һ��������������Ϊ15.3%��
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м����������
��2�����ݻ�ѧ����ʽ����������ɵ��Ȼ��Ƶ�������Ȼ�������ȫ��Ӧ��������Һ����=����������+���뷴Ӧ�����������-����������������Һ����������=��������Ȼ��Ƶ�����+���ɵ��Ȼ��Ƶ�������Ȼ�������������������ʽ���㼴�ɣ�
����⣺��Ӧ�й�����CO2������Ϊ����122.2g+25g-146.1g��×4=4.4g��
����뷴Ӧ��Na2CO3������Ϊx��
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 117 44
x y 4.4g
��
 ��
��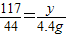
��֮�ã�x=10.6g��y=11.7g��
�������Na2CO3����Ϊ10.6g������NaCl������Ϊ11.7g��
����ȫ��Ӧ��������Һ����Ϊ��25g×4+16g-4.4g=111.6g��
����ȫ��Ӧ����Һ����������Ϊ��16g-10.6g+11.7g=17.1g��
����ȫ��Ӧ����Һ��������������Ϊ��
 ��15.3%��
��15.3%����ԭ������Na2CO3������Ϊ10.6g����Ӧ��������Һ��������������Ϊ15.3%��
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м����������

��ϰ��ϵ�д�
�����Ŀ





