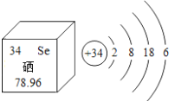
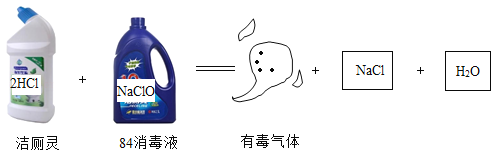
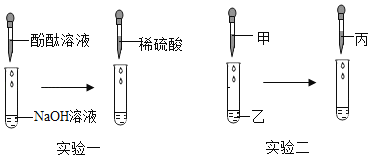
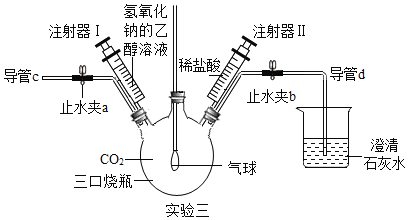
��Ŀ����
����Ŀ��Ϊ��֤Ba(OH)2�Ļ�ѧ��������ͼ��ʾ���ĸ�ʵ�飺
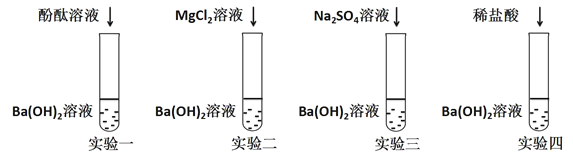
(1)ʵ��һ���ܹ��۲쵽������������____________��
(2)ʵ���������֤��Ba(OH)2�ܺ�ijЩ____________(���������)������ѧ��Ӧ��
(3)ʵ�����з�����Ӧ�Ļ�ѧ����ʽΪ____________��
(4)������ʵ�����֧�Թ��е��������ʵ���ͬһ���ձ�����ֻ�Ϻ���ˣ��õ���ɫ�������ҺA��ȡһ������ҺA����������Ba(OH)2��Һ����ֻ�õ���ɫ��Һ�����Ϸ�������ҺA�г���̪�⣬һ�������е�������____________��
���𰸡� ��Һ����ɫ��� �� Ba(OH)2+2HCl=BaCl2+2H2O NaCl��HCl��MgCl2
��������
��1�����������Ǽ�����Һ������ʹ��̪��Һ����ɫ��죻
��2�������֪��ʵ��2��3���������������η����ķ�Ӧ��
��3��ʵ��4��ϡ���������������Ӧ�����Ȼ�����ˮ����Ӧ����ʽΪBa(OH)2+2HCl=BaCl2+2H2O��
��4������ʵ�����֧�Թ��е��������ʵ���ͬһ���ձ�����ֻ�Ϻ���ˣ��õ���ɫ�������ҺA,����ҺA�м��������������õ���ɫ��Һ���������Һ���з�̪��˵����ҺA��һ����ϡ�������������������˷�Ӧ��������Һû�б�죬��ҺA����ϡ���ᣬ����Һ��һ����������������������һ������ʵ��3�����ɵ��������Ʒ�Ӧ�����Ȼ��ƣ���ʵ��2�е����ɵ�������þ��Ӧ�����Ȼ�þ����������������û�г������ɣ�˵����ҺA��һ��û�������ƣ�����ҺAһ�����е�������NaCl��HCl��MgCl2

 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�����Ŀ��ijѧϰС���ڰ���ʵ��Ա������ѧ�Լ�ʱ������һƿ��ǩ��ȱ����ɫ��Һ������ͼ��ʾ������ʵ��Ա������֪ԭƿ��Һ�е����ʿ����� NaHCO3��NaOH��Na2CO3��NaCl�е�һ�֣�����Ը��Լ��������벢����ʵ��̽����
��������⣩��ƿ�Լ���ʲô��Һ��
����������裩
�ײ��룺NaHCO3��Һ
�Ҳ��룺NaOH��Һ
�����룺Na2CO3��Һ
�����룺NaCl��Һ
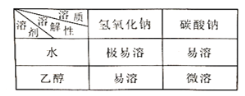
���������ϣ������������������Ϣ����
���� | NaHCO3 | NaOH | Na2CO3 | NaCl |
�������ܽ��/g | 9.6 | 109 | 21.5 | 36 |
������ϡ��Һ��pH | 9 | 13 | 11 | 7 |
��ʵ��̽��1��ȡƿ����Һ�������Թ��У��μӼ��η�̪��Һ����Һ��졣
��ʵ��̽��2����ȡƿ����Һ�������Թ��У��μ�������ϡ���ᣬ���������ݡ�
��ʵ�������ͨ����ʵ��̽��1����֪_____ͬѧ�IJ���һ������
��ͬѧ��ϸ�����������ʵ������Ϣ���������ѵIJ����������������_____��
��ͬѧ������ʵ��̽��2�����������ͬѧ��ʵ����ۣ���Ϊԭƿ��Һ��NaCO3��Һ��
��ʵ��̽��2���з�����Ӧ�Ļ�ѧ����ʽΪ_____��
���������ɣ�����Ϊ����ͬѧ������©������Ҫ��һ��ʵ��ȷ���������ֽ���������̽����
��ʵ��̽��3���ڣ�ʵ��̽��1���Թ��еĺ�ɫ��Һ�еμӹ����Ȼ�����Һ�����ԣ�����ַ�Ӧ���Թ�����Һ�Գʺ�ɫ�����а�ɫ����������
���ó����ۣ�ͨ����ʵ��̽��3����֤����ƿ�Լ�Ϊ_____��Һ�����Ѿ����ֱ��ʡ�
����˼����չ��ѧϰС�鷴˼����ƿ�Լ���Ϊ_____�����ʣ��ó��˸���Һ��ȷ�ı��淽������Ҫ��ȥ����Һ���ʲ��������õķ�����_____���û�ѧ��Ӧ����ʽ��ʾ����