��Ŀ����
����Խ����Ŀ�����������ѭ���ģ�
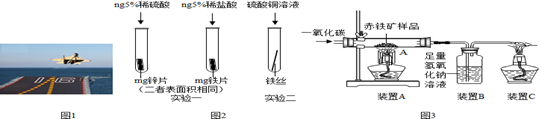
��1����ͭ�����������ʹ�õĽ�����Ʒ������ʱ�ڣ�������Ҫ���á�ʪ��ұ�𡱣��罫����������ͭ��Һ�У��÷�Ӧ�Ļ�ѧ����ʽΪ��_________����
��2�����ż����IJ��Ͻ��������������û�ԭ���ӽ������������н��仹ԭ���������磬��¯�������漰������ת���������£�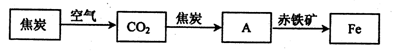
���У�����AΪ��_____�����ѧʽ����A��������е���������Ӧ�Ļ�ѧ����ʽΪ��_________����
��3��ÿ����Ϊ��ʴ���������⣩�����ϵĽ����൱���������20%��40%�����Dz��ö��ַ�����ֹ������ʴ�����磬����ϴ����������ɺ��ſ��Է�ֹ�������⣬��ԭ������_________����
��4�����Ľ�����Ա�����_________�����ǿ������������������Ʒȴ���бȽϺõĿ���ʴ���ܣ���ԭ������_________�����ñ�Ҫ�����ֺͻ�ѧ����ʽ˵������
��1��Fe + CuSO4 = FeSO4 + Cu
��2��CO ��Fe2O3 + 3CO 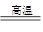 2Fe + 3CO2
2Fe + 3CO2
��3����ֹ����ˮ�Ӵ����������ɣ�
��4��ǿ ����������е�������Ӧ������һ�����ܵ���������Ĥ
���������������1���ڽ������˳���У���λ��ͭ��ǰ�棬�ܰ�����ͭ��Һ�е�ͭ�û�������
��2��������ԭ��������һ����̼��ԭ�������������Ͷ�����̼��
��3�����������������������ˮͬʱ�Ӵ���ΪΪ��ֹ�����⣬���ƻ����������������Ϳ����ȣ�
��4����������е�������Ӧ������һ�����ܵ���������Ĥ������ֹ���Ľ�һ��������
���㣺����

������;�㷺����ṹ�����ʵ��ǻ�ѧ����Ҫ�о����ݡ�
��1����ͼ������ԭ�ӽṹʾ��ͼ������˵������ȷ����_______��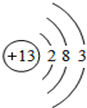
| A����ԭ���е�������Ϊ13 |
| B���ڻ���������ͨ����+3�� |
| C�����ǵؿ��к�������Ԫ�� |
| D���������������������������õĵ����� |

��������⡿��ҺA�е����ʿ�������Щ��
���������롿
�� ֻ��Zn��NO3��2 �� Zn��NO3��2��AgNO3
�� Zn��NO3��2��Cu��NO3��2 �� Zn��NO3��2��Cu��NO3��2��AgNO3
���������ۡ��������IJ�����_______�����ţ�����������_______��
��ʵ��̽����������ٳ�����ͨ������ʵ���ȷ������B�ijɷ֣��뽫�±���д������
| ʵ�鲽�� | ���� | ʵ����� |
| ȡ��������B���μ�_______ | �����ݲ��� | ����B����Cu ��Ag��_ |
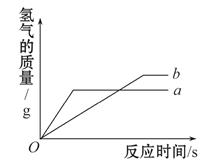
��3�����Ͳ������� Fe �۾��й㷺����;��������ͨ Fe �۸�����������Ӧ�����Ʊ�����������ͼ��ʾ��
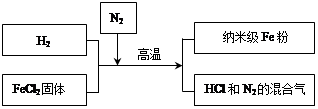
��ش��������⣺
������Fe���ڿ���������ȼ��ʵ����ͨ��N2��Ŀ����_______��
��д��H2��ԭFeCl2���û������Ļ�ѧ����ʽ_______��
���о���Ա��������Ƶõ����� Fe ����Ʒ�л��������� FeCl2���ʡ�ȡ��Ʒ20g���������������ᣬ�������� 0.7 g����������Ʒ�е��� Fe ��������������д��������̣���_______