��Ŀ����
�±���Ԫ�����ڱ���һ���֣�
��1��ԭ��������16��Ԫ�ط���Ϊ
��2��Ԫ�����ڱ��ķ��֣������������ѧ�ҵĹ��ͣ���д�����е�һλ
��3��ijԪ��M��ԭ�ӽṹʾ��ͼ����ͼ��ʾ�����Ԫ�ص�ԭ������ Ϊ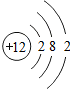
�� �ӣ�
| 1H 1.008 |
2He 4.003 | ||||||
| 3Li 6.941 |
4Be 9.012 |
5B 10.81 |
6C 12.01 |
7N 14.01 |
8O 16.00 |
9F 19.00 |
10Ne 20.18 |
| 11Na 22.99 |
12Mg 24.31 |
13Al 26.98 |
14Si 28.09 |
15P 30.97 |
16S 32.06 |
17Cl 35.45 |
18Ar 39.95 |
S
S
���ؿ��к������Ľ���Ԫ�صķ���ΪAl
Al
��BeԪ�ص����ԭ������Ϊ9.012
9.012
����2��Ԫ�����ڱ��ķ��֣������������ѧ�ҵĹ��ͣ���д�����е�һλ
�Ž��з�
�Ž��з�
��3��ijԪ��M��ԭ�ӽṹʾ��ͼ����ͼ��ʾ�����Ԫ�ص�ԭ������ Ϊ
12
12
����ԭ�Ӻ�����3
3
�����Ӳ㣬�ڻ�ѧ��Ӧ������ʧ
ʧ
����á���ʧ����
�� �ӣ�
��������1������Ԫ�����ڱ�����������ԭ��������12��Ԫ�ص�Ԫ�ط��ż��ɣ�
��2������1869�����Ž��з�Ϊ�����Ŀ�ѧ�ҷ�����Ԫ�����ڱ������н��
��3������ԭ�ӽṹʾ��ͼ���ص㣺����Ԫ�ص�����������һ������4����ʧȥ���������γ������ӣ��ǽ���Ԫ�ص�����������һ�����4���õ����ӣ��γ������ӣ����н��
��2������1869�����Ž��з�Ϊ�����Ŀ�ѧ�ҷ�����Ԫ�����ڱ������н��
��3������ԭ�ӽṹʾ��ͼ���ص㣺����Ԫ�ص�����������һ������4����ʧȥ���������γ������ӣ��ǽ���Ԫ�ص�����������һ�����4���õ����ӣ��γ������ӣ����н��
����⣺��1������Ԫ�����ڱ����16��Ԫ�ص�Ԫ�ط���Ϊ��S���ؿ��к������Ľ���Ԫ�������������ΪAl��BeԪ�ص����ԭ������Ϊ��9.012��
�ʴ�Ϊ��S�� Al��9.012��
��2������1869�����Ž��з�Ϊ�����Ŀ�ѧ�ҷ�����Ԫ�����ڱ������Ԫ�����ڱ��ķ��֣�����һλ��ѧ�ҵ����֣��Ž��з�
�ʴ𰸣��Ž��з�
��3����ԭ�ӽṹʾ��ͼ������Ԫ�ص�ԭ������Ϊ12����ԭ�Ӻ�����3�����Ӳ㣬������������2���ڻ�ѧ��Ӧ������ʧȥ������2�������γ��ȶ��ṹ��
�ʴ�Ϊ��12��3��ʧ��
�ʴ�Ϊ��S�� Al��9.012��
��2������1869�����Ž��з�Ϊ�����Ŀ�ѧ�ҷ�����Ԫ�����ڱ������Ԫ�����ڱ��ķ��֣�����һλ��ѧ�ҵ����֣��Ž��з�
�ʴ𰸣��Ž��з�
��3����ԭ�ӽṹʾ��ͼ������Ԫ�ص�ԭ������Ϊ12����ԭ�Ӻ�����3�����Ӳ㣬������������2���ڻ�ѧ��Ӧ������ʧȥ������2�������γ��ȶ��ṹ��
�ʴ�Ϊ��12��3��ʧ��
���������⿼��ѧ����Ԫ�����ڱ�֪ʶ�����������գ�������ע�⣺ԭ������=������=�˵����=�����������ǰ�������� ��ԭ���У�

��ϰ��ϵ�д�
�����Ŀ
�±���Ԫ�����ڱ���һ���֣�
��1��16��Ԫ�ص�Ԫ�ط���Ϊ ����Ԫ�ص�ԭ�ӽṹʾ��ͼ���ң���X����ֵ= ��
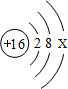
��2��8��Ԫ�غ�13��Ԫ����ɵĻ�����Ļ�ѧʽΪ ��
��3�����ñ��е�Ԫ�ط�������ɵķ�Ӧ��д������Ҫ��Ļ�ѧ����ʽ����ˮ���ɵĻ��Ϸ�Ӧ�� ��
| �� ���� |
IA | 0 | ||||||
| һ | 1H 1��008 |
��A | ��A | ��A | V A | ��A | ��A | 2He 4��003 |
| �� | 3Li 6��941 |
4Be 9��012 |
5B 10��81 |
6C 12��01 |
7N 14��01 |
8O 16��00 |
9F 19��00 |
10Ne 20��18 |
| �� | 11Na 22��99 |
12Mg 24��31 |
13Al 26��98 |
14Si 28��09 |
15P 30��97 |
16S 32��06 |
17Cl 35��45 |
18Ar 39��95 |
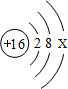
��2��8��Ԫ�غ�13��Ԫ����ɵĻ�����Ļ�ѧʽΪ
��3�����ñ��е�Ԫ�ط�������ɵķ�Ӧ��д������Ҫ��Ļ�ѧ����ʽ����ˮ���ɵĻ��Ϸ�Ӧ��


 ����m=
����m=
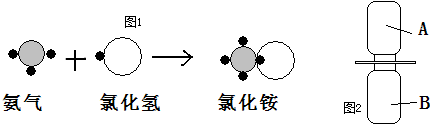
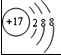 ��ʾ����
��ʾ���� ����ʾ��ԭ�ӣ��á�
����ʾ��ԭ�ӣ��á� ����ʾ��ԭ�ӣ��á�
����ʾ��ԭ�ӣ��á� ����ʾ��ԭ�ӣ�������Ӧ���̿���ͼ1��ʾ��
����ʾ��ԭ�ӣ�������Ӧ���̿���ͼ1��ʾ��