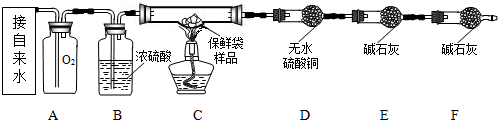
��Ŀ����
 ���������ʯ�ҡ��������ƺ��������ƵĻ����ڿ��������ױ��ʣ�ij�о�С���һֻ�������ġ���ʯ�ҡ���Ʒ�ijɷֽ���ʵ��̽����
���������ʯ�ҡ��������ƺ��������ƵĻ����ڿ��������ױ��ʣ�ij�о�С���һֻ�������ġ���ʯ�ҡ���Ʒ�ijɷֽ���ʵ��̽����[�������]
�����û�б��ʣ�ֻ���������ƺ��������ƣ�
������ֱ��ʣ����С�
�������ȫ���ʣ�ֻ����̼��ơ�̼���ƣ�
[ʵ��̽��]
| ���� | ���� | ���� |
�� |
�Թ���ڷ��̣��õ����ǵ�Һ�� | �Ʋ���Ʒ�к��� �����ƺ���������������һ�� �����ƺ���������������һ�� |
�� |
�����ܽ⣬������������ | д�������������ݵĻ�ѧ����ʽ CaCO3+2HCl=CaCl2+H2O+CO2�� CaCO3+2HCl=CaCl2+H2O+CO2�� |
�� |
������ɫ���� | д��������ɫ�����Ļ�ѧ����ʽ Ca��OH��2+Na2CO3=CaCO3��+2NaOH Ca��OH��2+Na2CO3=CaCO3��+2NaOH |
��1��������ʵ�������ƶϣ�����
II
II
��������2������ʯ�ҡ���Ʒ���Ƿ�һ������̼��ƣ�
��һ��
��һ��
���һ������һ����������˵�����ɣ�����ʯ����ͬʱ��Ca��OH��2��Na2CO3��ˮ��Ҳ��õ�̼���
����ʯ����ͬʱ��Ca��OH��2��Na2CO3��ˮ��Ҳ��õ�̼���
����3�����ü�ʯ�Ҹ����������
DE
DE
��A��HCl B��CO2 C��SO2 D��NH3 E��O2��
�����������ƿ�����ˮ������ѧ��Ӧ�����������ƶ����ʣ�ͬʱ�ų��������ȣ������ɵ��������ƻ������������̼������ѧ��Ӧ������̼��ƣ������������Ƽ������տ����е�ˮ���������⣬�������������ʶ�������ˮ�ԣ����Գ���������������������ƻ����������̼������ѧ��Ӧ����̼���ƶ����ʣ�
ʵ����ѡ���������Ҫ��ģ��������ѡ������ԭ���ǣ����Ը����������������������壬�����Ը�����������������������壮
ʵ����ѡ���������Ҫ��ģ��������ѡ������ԭ���ǣ����Ը����������������������壬�����Ը�����������������������壮
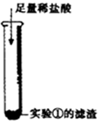
����⣺[ʵ��̽��]������Ϣ��ȡ��ʯ����Ʒ�����Թ��У�������ˮ�����ִ����Թ���ڷ��̣�˵����Ʒ��ˮ�Ӵ��ų��˴������ȣ�����Ʒ��Ӧ������ʯ�һ���������������һ����
��ʯ����Ʒ������ˮ��õ�����Һ��˵���в�����ˮ�����ʲ�����������ʯ�Һ��������Ƶı���������ɷ����ķ�Ӧ�Ļ�ѧ����ʽ�У�CaO+H2O=Ca��OH��2��Ca��OH��2+CO2=CaCO3��+H2O��2NaOH+CO2=Na2CO3+H2O��Na2CO3+Ca��OH��2=CaCO3��+2NaOH�������ƶϴ˲�����ˮ������Ϊ̼��ƣ����Բ�������̼���������ķ�Ӧ��
�ʷ�Ӧ�ķ���ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
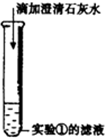
���ݢ�����Һ�е������ʯ��ˮ������ɫ������˵�������ܺ����ӽ�ϳɳ��������Ӵ��ڣ�˵����Һ�к��п����Ե�̼���ƣ�
�ʷ�Ӧ�ķ���ʽΪ��Ca��OH��2+Na2CO3=CaCO3��+2NaOH��
[С����˼��]��1���ۺϢ٢ڢ�����˵����ʯ��ֻ�Dz��ֱ���Ϊ̼���ƺ�̼��ƣ��ʲ���������
��2������ʯ�ҡ���Ʒ�ﲻһ������̼��ƣ���Ϊ����ʯ����ͬʱ��Ca��OH��2��Na2CO3��ˮ��Ҳ��õ�̼��ƣ�
��3���ɹ����������ƺ���������ɵĻ�����������������˸�������ڼ��Ը���������ܸ������֮��Ӧ���������壺�磮HCl��CO2��SO2 �ȣ���ѡ��Ϊ��DE��
�ʴ�Ϊ��[ʵ��̽��]�����ƺ���������������һ����CaCO3+2HCl=CaCl2+H2O+CO2����Ca��OH��2+Na2CO3=CaCO3��+2NaOH��
[С����˼��]��1����2����һ��������ʯ����ͬʱ��Ca��OH��2��Na2CO3��ˮ��Ҳ��õ�̼��ƣ���3��DE��
��ʯ����Ʒ������ˮ��õ�����Һ��˵���в�����ˮ�����ʲ�����������ʯ�Һ��������Ƶı���������ɷ����ķ�Ӧ�Ļ�ѧ����ʽ�У�CaO+H2O=Ca��OH��2��Ca��OH��2+CO2=CaCO3��+H2O��2NaOH+CO2=Na2CO3+H2O��Na2CO3+Ca��OH��2=CaCO3��+2NaOH�������ƶϴ˲�����ˮ������Ϊ̼��ƣ����Բ�������̼���������ķ�Ӧ��
�ʷ�Ӧ�ķ���ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
���ݢ�����Һ�е������ʯ��ˮ������ɫ������˵�������ܺ����ӽ�ϳɳ��������Ӵ��ڣ�˵����Һ�к��п����Ե�̼���ƣ�
�ʷ�Ӧ�ķ���ʽΪ��Ca��OH��2+Na2CO3=CaCO3��+2NaOH��
[С����˼��]��1���ۺϢ٢ڢ�����˵����ʯ��ֻ�Dz��ֱ���Ϊ̼���ƺ�̼��ƣ��ʲ���������
��2������ʯ�ҡ���Ʒ�ﲻһ������̼��ƣ���Ϊ����ʯ����ͬʱ��Ca��OH��2��Na2CO3��ˮ��Ҳ��õ�̼��ƣ�
��3���ɹ����������ƺ���������ɵĻ�����������������˸�������ڼ��Ը���������ܸ������֮��Ӧ���������壺�磮HCl��CO2��SO2 �ȣ���ѡ��Ϊ��DE��
�ʴ�Ϊ��[ʵ��̽��]�����ƺ���������������һ����CaCO3+2HCl=CaCl2+H2O+CO2����Ca��OH��2+Na2CO3=CaCO3��+2NaOH��
[С����˼��]��1����2����һ��������ʯ����ͬʱ��Ca��OH��2��Na2CO3��ˮ��Ҳ��õ�̼��ƣ���3��DE��
����������ͨ��ʵ���ҳ����������ʯ���Ƿ���ʵ�ʵ��̽�����ۺϿ����������ơ���ʯ�ҵ����ʣ��Լ���ˮ�������֮��Ļ�ѧ��Ӧ��

��ϰ��ϵ�д�
 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
�����Ŀ