��Ŀ����
����һ�ּ��Ե�ζҺ����̼���ƺ��Ȼ�����ɵ���Һ��Ϊ�˲ⶨ���Ե�ζҺ��̼���ƺ��Ȼ��Ƶ��������������������ʵ�鷽����
��ʵ��һ��ȡ���ݼ��Ե�ζҺ��100g���ֱ������뵽20g��40g��60gij�����У��������ʵ�����ݼ��±���������ܽ���Բ��ƣ���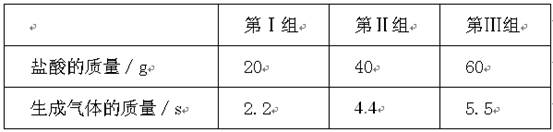
����ʵ�鼰�й����ݽ��з�������㣺
��1��100g���Ե�ζҺ��������ȫ��Ӧ�������������Ϊ g��
��2�����Ե�ζҺ��̼���Ƶ���������Ϊ����?��д��������̣�
��3��100g���Ե�ζҺ������ᷴӦ����ҺpH=7ʱ����Ҫ���������Ϊ g
��ʵ������������ڢ��鷴Ӧ�����Һ�м�AgNO3��Һ�����ʵ�����ݼ���ͼ: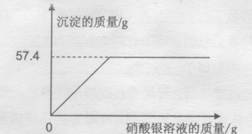
����ʵ�鼰�й����ݽ��з�����
��4��ijͬѧ��������Ե�ζҺ���Ȼ��Ƶ���������Ϊ20.5��(������С�����һλ)�������������������жϸü������Ƿ���ȷ���粻��ȷҪָ������ԭ��
��1��5��5(1��)
��2��(��4�֣���δ֪��������͵�λ��0��5�֣���ѧ����ʽ0��5�֣�������ϵʽ1�֣�x���1�֣�������������1��)
�⣺�������ᷴӦ��̼���Ƶ�����Ϊx��
Na2CO3+2HCl��2NaCl+CO2��+H2O
106 44
x 5.5g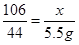 ���x=13.25g
���x=13.25g
���Ե�ζҺ��̼���Ƶ�����������(13.25g��100g)��100��=13.25��
�𣺼�����Һ��̼���Ƶ���������Ϊ13.25%��
��3��50(1��)
(4)����ȷ����̼���������ᷴӦ���ɵ��Ȼ��Ƶ�����û�м�ȥ(���̼���������ᷴӦ���ɵ��Ȼ��Ƶ�����Ҳ�����ȥ��)(��1��)
�������⿼����Ǻ��������ʵĻ�ѧ��Ӧ���йؼ��㡣
��1������ǰ����ʵ�����ݷ�����֪ÿ20��������ȫ��Ӧ����2.2�˶�����̼����60��������ȫ��ӦӦ����6.6�˶�����̼���ڵ�����ʵ���м���60������ֻ����5.5�˶�����̼��˵��������ʵ����������ʣ�̼࣬���Ʒ�Ӧ�꣬����100g���Ե�ζҺ��������ȫ��Ӧ�������������Ϊ5.5�ˡ�
��2�����ݶ�����̼�����������̼���Ƶ��������ٸ��� ��100%��������Ե�ζҺ��̼���Ƶ�����������
��100%��������Ե�ζҺ��̼���Ƶ�����������
�������ᷴӦ��̼���Ƶ�����Ϊx
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 44
x 5.5g
106:44 =x:5.5g
x=13.25g
���Ե�ζҺ��̼���Ƶ���������= ��100%=13.25%
��100%=13.25%
��3������ʵ�����ݷ�����֪ÿ����1.1�˶�����̼�μӷ�Ӧ�����������Ϊ10�ˣ����ڹ�����5.5�˶�����̼���ʲμӷ�Ӧ�����������Ϊ50�ˡ�
��4���ȼ�������Ե�ζҺ���Ȼ��Ƶ������������жϸü������Ƿ�ȷ��Ȼ����ݼ�������������ԭ������Ե�ζҺ���Ȼ��Ƶ����������ķ����ǣ�����Ϊ�Ȼ���������ʣ����������������Ӧ���ɵ��Ȼ������Ȼ�������������Ӧ���ɵ��Ȼ����������ʣ�����������ʵ�����������ʣ�����������ʵ�������������ᷴӦ���ɵ��Ȼ���������������������Ȼ��Ʒ�Ӧ���ɵ��Ȼ����������������Ȼ��ƺ���������Ӧ�Ļ�ѧ����ʽ�������Ȼ�������������Ȼ��Ƶ����������Ȼ��ư�����Ӧ���ɵ��Ȼ��ƺ�ԭ������е��Ȼ��ƣ������Ȼ��Ƶ�����-��Ӧ���ɵ��Ȼ��Ƶ�������Ϊ���Ե�ζҺ���Ȼ��Ƶ������������� ��100%����������Ե�ζҺ���Ȼ��Ƶ�����������
��100%����������Ե�ζҺ���Ȼ��Ƶ�����������
��20�����������ʵ�����Ϊy
Na2CO3+2HCl=2NaCl+CO2��+H2O
73 44
y 2.2g
73��44 =y��2.2g
y=3.65g
�������������ʵ���������= ��100%=18.25%
��100%=18.25%
�ʵڢ��鷴Ӧ�����Һ�к��Ȼ��������=10g��18.25%=1.825g
������ᷴӦ���ɵ��Ȼ���������Ϊz
HCl+AgNO3�TAgCl��+HNO3
36.5 143.5
1.825g z
36.5��143.5=1.825g��z
z=7.175g
����Ȼ��Ʒ�Ӧ���ɵ��Ȼ���������=57.4g-7.175g=50.225g
���Ȼ��Ƶ�������Ϊm
NaCl+AgNO3�TAgCl��+NaNO3
58.5 143.5
m 50.225g
58.5: 143.5 =" m:50.225g"
m=20.5g
�跴Ӧ���ɵ��Ȼ��Ƶ�����Ϊn
Na2CO3+2HCl=2NaCl+CO2��+H2O
117 44
n 5.5g
117:44="n:5.5g"
n=14.625g
���Ե�ζҺ���Ȼ��Ƶ�����=20.5g-14.625g=5.875g
���Ե�ζҺ���Ȼ��Ƶ���������= ��100%=5.875%
��100%=5.875%
����ü��������ԣ��ü�����Ϊ20.5%���������������֪����̼���������ᷴӦ���ɵ��Ȼ��Ƶ�����û�м�ȥʱ����Ľ����20.5%��

����һ�ּ��Ե�ζҺ����̼���ƺ��Ȼ�����ɵ���Һ��Ϊ�˲ⶨ���Ե�ζҺ��̼���Ƶ�����������ijͬѧ����������ʵ�飺ȡ���ݼ��Ե�ζҺ��100g���ֱ������뵽20g��40g��60gijŨ�ȵ�ϡ�����У��������ʵ�����ݼ��±���������ܽ���Բ��ƣ���
| �ڢ��� | �ڢ��� | �ڢ��� | |
| ϡ���������/g | 20 | 40 | 60 |
| �������������/s | 2.2 | 4.4 | 5.5 |
��1��100g���Ե�ζҺ��ϡ������ȫ��Ӧ�������������Ϊ______g��
��2�����Ե�ζҺ��̼���Ƶ���������Ϊ���٣�
��3��100g���Ե�ζҺ���ϡ���ᷴӦ����ҺpH=7ʱ������ϡ���������Ϊ���ٿˣ�
 ��2011?��ɽ������һ�ּ��Ե�ζҺ����̼���ƺ��Ȼ�����ɵ���Һ��Ϊ�˲ⶨ���Ե�ζҺ��̼���ƺ��Ȼ��Ƶ��������������������ʵ�鷽����
��2011?��ɽ������һ�ּ��Ե�ζҺ����̼���ƺ��Ȼ�����ɵ���Һ��Ϊ�˲ⶨ���Ե�ζҺ��̼���ƺ��Ȼ��Ƶ��������������������ʵ�鷽����