��Ŀ����
 ��Ԫ�ط��Ż�ѧʽ��ʾ��
��Ԫ�ط��Ż�ѧʽ��ʾ����1��2����ԭ��
2Fe
2Fe
����2��4�������
4H2
4H2
����3��3��������
3Al3+
3Al3+
����4��������ͭ
Cu2O
Cu2O
����5�������������Ԫ����+7��
K
O4
| +7 |
| Mn |
K
O4
��| +7 |
| Mn |
��6��������
Fe2��SO4��3
Fe2��SO4��3
����7��2�����������
2NO3-
2NO3-
����8�������ӵĽṹʾ��ͼ

��9��ͼ��6������
��ԭ���������6������
��ԭ���������6������
�����������⿼�黯ѧ��������弰��д������ؼ��Ƿ��廯ѧ����������Ķ����Ƿ��ӡ�ԭ�ӡ����ӡ����ṹʾ��ͼ�����ǻ��ϼۣ������ڻ�ѧ����ǰ������λ�ü����ʵ��ļ������������ر��������壬���ܸ������ʻ�ѧʽ����д������ȷ��д���ʵĻ�ѧʽ��
����⣺��1��ԭ�ӵı�ʾ��������Ԫ�ط�������ʾһ��ԭ�ӣ���ʾ�����ԭ�ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�����2����ԭ�ӣ��Ϳɱ�ʾΪ 2Fe��
�ʴ�Ϊ��2Fe��
��2�����ӵı�ʾ��������ȷ��д���ʵĻ�ѧʽ�����������˫ԭ�ӷ��ӣ��ɱ�ʾΪ��H2����ʾ����÷��ӣ������仯ѧʽǰ������Ӧ�����֣�����4������ӿɱ�ʾΪ4H2��
�ʴ�Ϊ��4H2��
��3�����ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�����3�������ӿɱ�ʾΪ��3Al3+��
�ʴ�Ϊ��3Al3+��
��4��������ͭ��˵������������Ԫ�غ�ͭԪ����ɣ���Ԫ����+2�ۣ�ͭԪ�����������ϼۣ�+1��+2���ͼ�̬��ͭԪ����ɵĻ����������ͭ������������ͭ��ͭԪ����+1�ۣ���������ǰ�����ۺ��滯�ϼ���ֵ��д�����ʵĻ�ѧʽΪ��
Cu2O��
�ʴ�Ϊ��Cu2O��
��5��Ԫ�ػ��ϼ۵ı�ʾ������ȷ��������������Ҫ�����Ԫ�صĻ��ϼۣ�Ȼ�����仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں����Ը����������Ԫ����+7�ۿɱ�ʾΪ��K
O4��
�ʴ�Ϊ��K
O4��
��6������������Ԫ�غ��������ɣ���Ԫ����+3�ۣ��������-2�ۣ���������ǰ�����ۺ��滯�ϼ���ֵ��д�����ʵĻ�ѧʽΪ��Fe2��SO4��3��
�ʴ�Ϊ��Fe2��SO4��3��
��7���������ӵı�ʾ������д��������������ɶ��ԭ����ɵ����ӣ������1����λ�ĸ���ɣ���ʾΪNO3-������ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�����2����������ӿɱ�ʾΪ��2NO3-��
�ʴ�Ϊ��2NO3-��
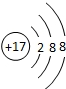
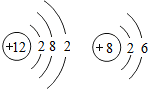
��8������17��Ԫ�أ�����������=ԭ������=����������������������������17������ԭ��������������7�����õ�1�����Ӵﵽ8�����ȶ��ṹ�����������ӵĽṹʾ��ͼΪ�� ��
��
�ʴ�Ϊ�� ��
��
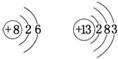
��9����ͼ��6��ʾ��ԭ���������6�����ӣ�
�ʴ�Ϊ����ԭ���������6�����ӣ�
�ʴ�Ϊ��2Fe��
��2�����ӵı�ʾ��������ȷ��д���ʵĻ�ѧʽ�����������˫ԭ�ӷ��ӣ��ɱ�ʾΪ��H2����ʾ����÷��ӣ������仯ѧʽǰ������Ӧ�����֣�����4������ӿɱ�ʾΪ4H2��
�ʴ�Ϊ��4H2��
��3�����ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�����3�������ӿɱ�ʾΪ��3Al3+��
�ʴ�Ϊ��3Al3+��
��4��������ͭ��˵������������Ԫ�غ�ͭԪ����ɣ���Ԫ����+2�ۣ�ͭԪ�����������ϼۣ�+1��+2���ͼ�̬��ͭԪ����ɵĻ����������ͭ������������ͭ��ͭԪ����+1�ۣ���������ǰ�����ۺ��滯�ϼ���ֵ��д�����ʵĻ�ѧʽΪ��
Cu2O��
�ʴ�Ϊ��Cu2O��
��5��Ԫ�ػ��ϼ۵ı�ʾ������ȷ��������������Ҫ�����Ԫ�صĻ��ϼۣ�Ȼ�����仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں����Ը����������Ԫ����+7�ۿɱ�ʾΪ��K
| +7 |
| Mn |
�ʴ�Ϊ��K
| +7 |
| Mn |
��6������������Ԫ�غ��������ɣ���Ԫ����+3�ۣ��������-2�ۣ���������ǰ�����ۺ��滯�ϼ���ֵ��д�����ʵĻ�ѧʽΪ��Fe2��SO4��3��
�ʴ�Ϊ��Fe2��SO4��3��
��7���������ӵı�ʾ������д��������������ɶ��ԭ����ɵ����ӣ������1����λ�ĸ���ɣ���ʾΪNO3-������ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�����2����������ӿɱ�ʾΪ��2NO3-��
�ʴ�Ϊ��2NO3-��
��8������17��Ԫ�أ�����������=ԭ������=����������������������������17������ԭ��������������7�����õ�1�����Ӵﵽ8�����ȶ��ṹ�����������ӵĽṹʾ��ͼΪ��
 ��
���ʴ�Ϊ��
 ��
����9����ͼ��6��ʾ��ԭ���������6�����ӣ�
�ʴ�Ϊ����ԭ���������6�����ӣ�
������������Ҫ����ѧ���Ի�ѧ�������д��������������Ŀ��ƼȰ����Ի�ѧ����������˽⣬�ֿ�����ѧ���Ի�ѧ���ŵ���д������ԭ�ӵĶ�����ɣ�ԭ����ԭ�Ӻ˺ͺ�����ӹ��ɣ��������Ӻ͵��ӡ�ԭ��������ȣ�

��ϰ��ϵ�д�
�����Ŀ


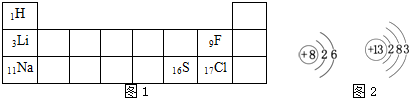
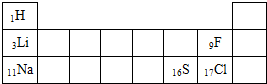 ��3���ϱ�����Ԫ�ص�ÿ��ԭ����Ҫ�õ�
��3���ϱ�����Ԫ�ص�ÿ��ԭ����Ҫ�õ�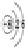


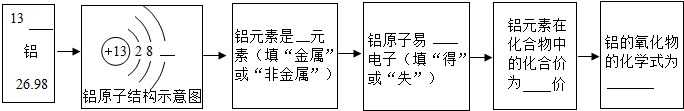
 �±���Ԫ�����ڱ���һ���֣����ֱ�ʾ��ӦԪ�ص�ԭ��������������Ԫ�ط��Ž�����Ԫ�����������Ӧλ�ã�
�±���Ԫ�����ڱ���һ���֣����ֱ�ʾ��ӦԪ�ص�ԭ��������������Ԫ�ط��Ž�����Ԫ�����������Ӧλ�ã�