��Ŀ����
��10��������16������15�֣���ͼ�ǻ�ѧʵ������ȡ����ij���װ�á�
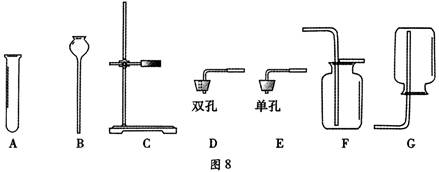
��1����д��ͼ�б��Тٵ��������ƣ� ��
��2���������������dz��н̲��г��ֵĻ�ѧ���ʣ���һ�������£������Բ���������
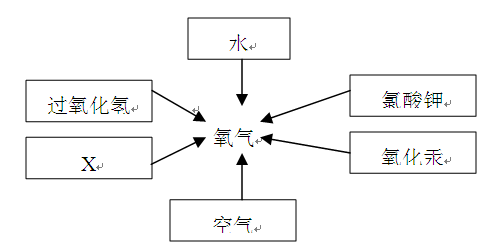
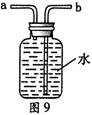
����д����ͼ������X������ ��ʵ�����ø�ҩƷ��ȡ�����Ļ�ѧ����ʽΪ�� ����Ҫ��ȡ�ϴ�������������ʵ��װ��Ӧѡ �� �����ţ���
��3��ʵ������B��Cװ�ÿ���ȡ ���壨��һ�ּ��ɣ�����ȡ������Ļ�ѧ����ʽΪ ��װ��B��C�Ƚϣ�B���ŵ��ǣ� ��дһ�㣩��
��4���������ƣ�Na2O2�������ڳ�������ˮ��ӦҲ���Բ���������ͬʱ�����������ơ�д���÷�Ӧ�Ļ�ѧ����ʽ�� ��

��1����д��ͼ�б��Тٵ��������ƣ� ��
��2���������������dz��н̲��г��ֵĻ�ѧ���ʣ���һ�������£������Բ���������

����д����ͼ������X������ ��ʵ�����ø�ҩƷ��ȡ�����Ļ�ѧ����ʽΪ�� ����Ҫ��ȡ�ϴ�������������ʵ��װ��Ӧѡ �� �����ţ���
��3��ʵ������B��Cװ�ÿ���ȡ ���壨��һ�ּ��ɣ�����ȡ������Ļ�ѧ����ʽΪ ��װ��B��C�Ƚϣ�B���ŵ��ǣ� ��дһ�㣩��
��4���������ƣ�Na2O2�������ڳ�������ˮ��ӦҲ���Բ���������ͬʱ�����������ơ�д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
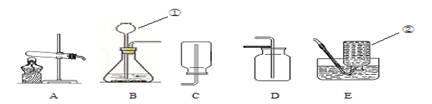
��1������ƿ
��2��������أ� 2KMnO4 K2MnO4��MnO2��O2���� A��E����E��A��
K2MnO4��MnO2��O2���� A��E����E��A��
��3��O2����CO2��H2��
2H2O2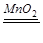 2H2O��O2����CaCO3��2HCl��CaCl2��H2O��CO2����
2H2O��O2����CaCO3��2HCl��CaCl2��H2O��CO2����
��Zn��H2SO4��ZnSO4+H2��
���Ʒ�Ӧ���ʣ����ԼҩƷ��
��4��2Na2O2��2H2O��4NaOH��O2��
��2��������أ� 2KMnO4
 K2MnO4��MnO2��O2���� A��E����E��A��
K2MnO4��MnO2��O2���� A��E����E��A����3��O2����CO2��H2��
2H2O2
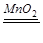 2H2O��O2����CaCO3��2HCl��CaCl2��H2O��CO2����
2H2O��O2����CaCO3��2HCl��CaCl2��H2O��CO2������Zn��H2SO4��ZnSO4+H2��
���Ʒ�Ӧ���ʣ����ԼҩƷ��
��4��2Na2O2��2H2O��4NaOH��O2��
����������ʵ������ȡ���������ԭ����װ�á��ռ���������������嶼�����ù�Һ������װ����ȡ��ʵ���������������ַ���������װ�������֣���������ͺ�Һ�����ͣ��ռ�װ�ø���������ܶȺ��ܽ���ѡ��
�⣺��1���������Ƽ���ƿ
��2����ʵ�����������ļ��ַ�����֪����XΪ������أ���������������Ļ�ѧ����ʽΪ��
2KMnO4 K2MnO4��MnO2��O2��������������ˮ��������ˮ���ռ����ܶȱȿ�����Ҳ���������ſ������ռ�������ˮ���ռ��ϴ�����װ��ѡ��ΪAE��
K2MnO4��MnO2��O2��������������ˮ��������ˮ���ռ����ܶȱȿ�����Ҳ���������ſ������ռ�������ˮ���ռ��ϴ�����װ��ѡ��ΪAE��
��3��װ��B��C�ǹ�Һ������װ�ã���Ӧ��Ϊ������Һ�壬����Ϊ���£���˿�����˫��ˮ��������ʵ�����������Ͷ�����̼�����ԣ���ѧ����ʽΪ��2H2O2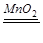 2H2O��O2����CaCO3+2HCl=CaCl2+H2O+CO2����Zn+H2SO4=ZnSO4+H2����װ��B�õ��Ƿ�Һ©���ɿ��Ʒ�Ӧ�ٶȣ�
2H2O��O2����CaCO3+2HCl=CaCl2+H2O+CO2����Zn+H2SO4=ZnSO4+H2����װ��B�õ��Ƿ�Һ©���ɿ��Ʒ�Ӧ�ٶȣ�
��4�����ݷ�Ӧ�����������д����ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2��
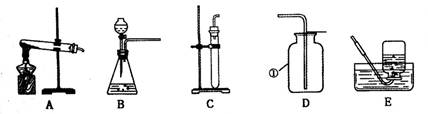
�ʴ�Ϊ����1������ƿ
��2��������أ�2KMnO4 K2MnO4��MnO2��O2����A����E���� E����A��
K2MnO4��MnO2��O2����A����E���� E����A��
��3��O2����CO2��H2��2H2O2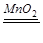 2H2O��O2����CaCO3+2HCl=CaCl2+H2O+CO2����Zn+H2SO4=ZnSO4+H2������
2H2O��O2����CaCO3+2HCl=CaCl2+H2O+CO2����Zn+H2SO4=ZnSO4+H2������
��4��2Na2O2+2H2O=4NaOH+O2����
�⣺��1���������Ƽ���ƿ
��2����ʵ�����������ļ��ַ�����֪����XΪ������أ���������������Ļ�ѧ����ʽΪ��
2KMnO4
 K2MnO4��MnO2��O2��������������ˮ��������ˮ���ռ����ܶȱȿ�����Ҳ���������ſ������ռ�������ˮ���ռ��ϴ�����װ��ѡ��ΪAE��
K2MnO4��MnO2��O2��������������ˮ��������ˮ���ռ����ܶȱȿ�����Ҳ���������ſ������ռ�������ˮ���ռ��ϴ�����װ��ѡ��ΪAE����3��װ��B��C�ǹ�Һ������װ�ã���Ӧ��Ϊ������Һ�壬����Ϊ���£���˿�����˫��ˮ��������ʵ�����������Ͷ�����̼�����ԣ���ѧ����ʽΪ��2H2O2
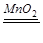 2H2O��O2����CaCO3+2HCl=CaCl2+H2O+CO2����Zn+H2SO4=ZnSO4+H2����װ��B�õ��Ƿ�Һ©���ɿ��Ʒ�Ӧ�ٶȣ�
2H2O��O2����CaCO3+2HCl=CaCl2+H2O+CO2����Zn+H2SO4=ZnSO4+H2����װ��B�õ��Ƿ�Һ©���ɿ��Ʒ�Ӧ�ٶȣ���4�����ݷ�Ӧ�����������д����ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2��
�ʴ�Ϊ����1������ƿ
��2��������أ�2KMnO4
 K2MnO4��MnO2��O2����A����E���� E����A��
K2MnO4��MnO2��O2����A����E���� E����A����3��O2����CO2��H2��2H2O2
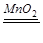 2H2O��O2����CaCO3+2HCl=CaCl2+H2O+CO2����Zn+H2SO4=ZnSO4+H2������
2H2O��O2����CaCO3+2HCl=CaCl2+H2O+CO2����Zn+H2SO4=ZnSO4+H2��������4��2Na2O2+2H2O=4NaOH+O2����

��ϰ��ϵ�д�
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
�����Ŀ

 (2)���ø��������ȡ�������������Ӿƾ����⣬����Ҫ��ʵ����Ʒ�� ���÷�Ӧ�Ļ�ѧ����ʽ�� ��
(2)���ø��������ȡ�������������Ӿƾ����⣬����Ҫ��ʵ����Ʒ�� ���÷�Ӧ�Ļ�ѧ����ʽ�� ��