��Ŀ����
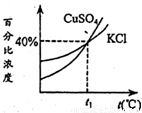
 ��ͼ��KCl��CuSO4�������ʵı�����Һ�������ٷֱ�Ũ�����¶ȱ仯���ߣ��ֱַ���50g KCl��80g CuSO4���壨�����ᾧˮ���м�ˮ150g�������ܽ⣬Ȼ��ֱ�������50gˮ����ȴ��t1�棬����������ȷ���ǣ�������
��ͼ��KCl��CuSO4�������ʵı�����Һ�������ٷֱ�Ũ�����¶ȱ仯���ߣ��ֱַ���50g KCl��80g CuSO4���壨�����ᾧˮ���м�ˮ150g�������ܽ⣬Ȼ��ֱ�������50gˮ����ȴ��t1�棬����������ȷ���ǣ�����������������ͭ�ڴ���Һ������ʱ���Ծ������ʽ���������Կ��Ծݴ˽������ͭ��ˮ��Ӧ��������ͭ����Ļ�ѧ����ʽ���м��㣬���������жϼ��ɣ�
����⣺��������50gˮ������������ͭ������Ϊx��������Ϊx������ͭ����ˮ������Ϊy����
CuSO4+5H2O�TCuSO4?5H2O
160 90
x y
=
��ã�y=
��100%=40%
��ã�x=51.6g
���Դ�ʱ����ͭ��Һ�к��е�����ͭ������Ϊ��80g-51.6g=29.4g
����������ͭ���������Ϊ��51.6g+
=80.6g
A����������������֪�����Ȼ������¶�Ϊt1��ʱ�ı�����Һ����������Ϊ40%�����ܼ�������Ϊ100g�����Ը��¶����Ȼ��ص��ܽ��Ϊ40g����������ͭ���ܽ��Ҫ����40g����A����
B����������������֪������������ͭ���������Ϊ80.6g����B����
C����ͼ�п���֪���Ȼ��غ�����ͭ�������ٷֱȶ�Ϊ40%����C����
D������A�еĽ�����֪�����¶�Ϊt1��ʱ�Ȼ��ص��ܽ��Ϊ40g����������ˮ������50g��Ҫ�����Ȼ���10g����D��ȷ��
��ѡD��
CuSO4+5H2O�TCuSO4?5H2O
160 90
x y
| 160 |
| x |
| 90 |
| y |
��ã�y=
| 9x |
| 16 |
| 80g-x | ||
150g-50g-
|
��ã�x=51.6g
���Դ�ʱ����ͭ��Һ�к��е�����ͭ������Ϊ��80g-51.6g=29.4g
����������ͭ���������Ϊ��51.6g+
| 9��51.6g |
| 16 |
A����������������֪�����Ȼ������¶�Ϊt1��ʱ�ı�����Һ����������Ϊ40%�����ܼ�������Ϊ100g�����Ը��¶����Ȼ��ص��ܽ��Ϊ40g����������ͭ���ܽ��Ҫ����40g����A����
B����������������֪������������ͭ���������Ϊ80.6g����B����
C����ͼ�п���֪���Ȼ��غ�����ͭ�������ٷֱȶ�Ϊ40%����C����
D������A�еĽ�����֪�����¶�Ϊt1��ʱ�Ȼ��ص��ܽ��Ϊ40g����������ˮ������50g��Ҫ�����Ȼ���10g����D��ȷ��
��ѡD��
���������������Ŀʱ�����ȣ�Ҫ��Ǻ����������������������йؼ��㣬������Һ�Ͳ�������Һ�ĸ�����ת�䷽�����Լ����ʵ��ܽ�ͽᾧ�ķ��������֪ʶ��Ȼ��������ͼ����Ϣ�������ѧ�����֪ʶ�ͼ���ϸ�ĵؽ���̽�����������������Ŀ��Ҫ������ؽ���ѡ����ɣ�

��ϰ��ϵ�д�
 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
�����Ŀ
 KCl�dz��õĻ��ʣ���ͼ��KCl���ܽ�����ߣ���ش�
KCl�dz��õĻ��ʣ���ͼ��KCl���ܽ�����ߣ���ش� ��2013?��Ϫһģ����ͼ��KCl��KNO3�������ʵ��ܽ�����ߣ��Իش��������⣺
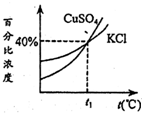
��2013?��Ϫһģ����ͼ��KCl��KNO3�������ʵ��ܽ�����ߣ��Իش��������⣺ ��ͼ��KCl��CuSO4�������ʵı�����Һ�������ٷֱ�Ũ�����¶ȱ仯���ߣ��ֱַ���50g KCl��80g CuSO4���壨�����ᾧˮ���м�ˮ150g�������ܽ⣬Ȼ��ֱ�������50gˮ����ȴ��t1�棬����������ȷ����
��ͼ��KCl��CuSO4�������ʵı�����Һ�������ٷֱ�Ũ�����¶ȱ仯���ߣ��ֱַ���50g KCl��80g CuSO4���壨�����ᾧˮ���м�ˮ150g�������ܽ⣬Ȼ��ֱ�������50gˮ����ȴ��t1�棬����������ȷ����