��Ŀ����
����Ŀ����֪Na2CO3��ˮ��Һ�ʼ��ԡ����ձ���12gNa2CO3��NaCl��������Ƴ�62.4g����Һ������Һ���μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ��ͼ����ش�����:
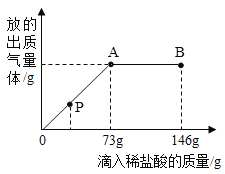
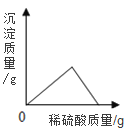
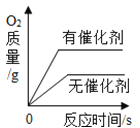
��1�����μ�ϡ������ͼ��p��ʱ���ձ�����Һ��pH_____7(ѡ��>��=��<);��ʱ��Һ����������______(�ѧʽ)��
��2�����μ�ϡ������ͼ��A��ʱ�����ʱ��Һ��������������?_______
���𰸡�> Na2CO3 NaCl 10%
��������
�⣨1�����μ�ϡ������ͼ��p��ʱ���ձ���̼������ʣ�࣬��Һ��pH��7��̼���������ᷴӦ�����Ȼ�����Һ�Ͷ�����̼���壬��ʱ��Һ���������ʣ�̼���ƺ��Ȼ��ƣ�
��2����:�赱�μ�ϡ������ͼ��A��ʱ����Ӧ���ɵ��Ȼ�������Ϊ����x���μӷ�Ӧ��̼���Ƶ�����Ϊy��������̼������Ϊz��
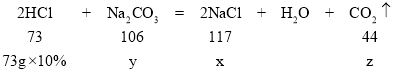
![]() x=11.7g
x=11.7g
![]() y=10.6g
y=10.6g
![]() z=4.4g
z=4.4g
��Ӧ����Һ��������������:![]()
��:���μ�ϡ������ͼ��A��ʱ����Ӧ����Һ��������������Ϊ10%��

����Ŀ��һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ��Ҫ��ͬѧ�ǽ���̽����ȷ����ƿ������ʲô��Һ��
��������룩С��������������ֲ��룺
����٣�����þ��Һ��
����ڣ���������Һ��
����ۣ��������Һ��
����ܣ�������Һ��
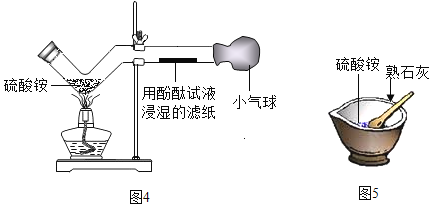
���������ϣ���NH4��2SO4��ˮ��Һ�����ԣ�
�ڳ����£�������ʵ��ܽ�����£�
���� | MgSO4 | Na2SO4 | ��NH4��SO4 | H2SO4 |
�ܽ�� | 35.1g | 19.5g | 75.4g | ��ˮ����������� |
��ʵ��̽����
��1��С����Ϊ����______��������ԭ����_______��
��2��Ϊȷ���������ֲ����Ƿ���ȷ��С��ͬѧ��������̽����
ʵ����� | ʵ������ | ʵ����� |
��ȡ����Һ�������Թ��У������еμӼ���_____��Һ | ��Һ��û�а�ɫ�������� | ����ٲ����� |
���ò�����պȡ����ԭ��Һ����pH��ֽ�ϣ����ͱ���ɫ������ | ��Һ��pHֵС��7 | ����ܳ��� |
С����ΪС��ʵ������ڲ���֤���ò���ܳ�����������_________��
��3���������ʵ�鷽����ȷ�ϸ���Һ���������Һ�����ʵ�鱨�棺
ʵ����� | ʵ������ | ʵ����� |
ȡ��������Һ���Թ��У������м���________ | _________ | ����۳��� |
����չӦ�ã�����̽�������Ҷ��㵹Һ��ʱ����ǩҪ�������������µ���ʶ������������ԭ����_________��