��Ŀ����
���ȡһ������þ�ۺ�ͭ�۵Ļ�������ձ��У��������зִμ����������ͬһϡ���ᡣʵ��������й����ʵ������������ⶨ��¼���±���
��ش��������⣺
��1�������ȡþ�ۺ�ͭ�ۻ���������Ϊ g��
��2��ʵ��������������Һ�����ʵĻ�ѧʽΪ ��
��3������ϡ���������ʵ���������Ϊ ��
��4����Ӧ��������Һ���������Ƕ��٣�Ҫ�н����̣������ȷ��0.01g����
| | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ����ϡ��������� | 35g | 35g | 35g | 35g |
| ʣ���������� | 8.6g | 7.4g | 6.2g | 5.6g |
��1�������ȡþ�ۺ�ͭ�ۻ���������Ϊ g��
��2��ʵ��������������Һ�����ʵĻ�ѧʽΪ ��
��3������ϡ���������ʵ���������Ϊ ��
��4����Ӧ��������Һ���������Ƕ��٣�Ҫ�н����̣������ȷ��0.01g����
��1��9.8 ��2��MgSO4��H2SO4 (3)14% (4)143.85g
�����������1�������з�Ӧ�����ݿ�֪��ÿ�μ���35gϡ���ᣬ����Ӧ������1.2g���������ȡþ�ۺ�ͭ�ۻ���������Ϊ8.6+1.2=9.8g��
��2��þ�Ľ������ǿ���⣬�������ᷴӦ����ͭ���������ᷴӦ��ʵ��������������Һ�����ʵĻ�ѧʽΪMgSO4��H2SO4��
��3���裺35gϡ�����к������������ΪX����Ӧ��������������ΪY��
H2SO4+Mg= MgSO4+ H2
98 24 2
X 1.2g Y
98:24=X��1.2g
X=4.9g
24:2=1.2g��Y
Y=0.2g
����ϡ���������ʵ���������Ϊ��4.9/35����100%=14%��
��4����þȫ����Ӧ���������������ΪY��
H2SO4+Mg= MgSO4+ H2��
24 2
4.2g Y
24:2=4.2g��Y
Y=0.35g
��Ӧ��������Һ���������ǣ�35��4+1.2������0.6-0.35=143.85g
�𣺣�1�������ȡþ�ۺ�ͭ�ۻ���������Ϊ9.8g��
��2��ʵ��������������Һ�����ʵĻ�ѧʽΪMgSO4��H2SO4 ��
��3������ϡ���������ʵ���������Ϊ14%��
��4����Ӧ��������Һ����������143.85g
���������ݻ�ѧ����ʽ���㣬ע���衢д���ҡ��С��⡢��Ľ��ⲽ�衣

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

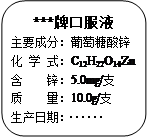

 Si+ 4HCl�������Ҫ���56g�裨Si����������Ҫ�������ٿˣ�
Si+ 4HCl�������Ҫ���56g�裨Si����������Ҫ�������ٿˣ�
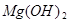 +2HCl=
+2HCl= +H2O��
+H2O��