��Ŀ����
����Ŀ��ijУ��ѧʵ���ҷ�ҺͰ���ռ������д���FeSO4��CuSO4�ķ�ˮ����ֱ���ŷŵ���ˮ����������ؽ�����Ⱦ������˷ѡ�����(2)���ͬѧ�������ø�ѧ�����������������˳��������������йػ�ѧ֪ʶ�Է�ˮ���д�����
(1)��ϰ�����������˳��������ո��зֱ������Ӧ��Ԫ�ط��š�
![]() K Ca Na Mg Al___________Fe Sn Pb (H) Cu Hg_____________Pt Au
K Ca Na Mg Al___________Fe Sn Pb (H) Cu Hg_____________Pt Au
������������
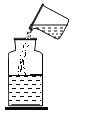
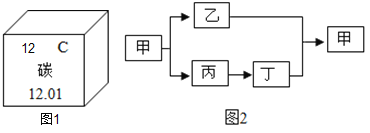
(2)��ƻ����������������ͭ��ʵ�鷽�����£�

��ش��������⣺
������ʵ������ж�β�ȡ���ˣ��ò����õ��IJ����������ձ���_______________________�Ͳ�������
�ڽ���X��__________________________��������ˮ������Ӧ�Ļ�ѧ����ʽΪ________________________________��
����ҺY��__________________________��������ɳ�ȥͭ�б�ͭ���õĽ������ʡ�
���𰸡�Zn Ag ©�� �� Fe+CuSO4=FeSO4+Cu ����ͭ��ϡ����
��������
(1)�����˳�����K Ca Na Mg Al Zn Fe Sn Pb H Cu Hg Ag Pt Au���ʿհ״��������룺Zn��Ag��
(2) ������ʵ������ж�β�ȡ���ˣ��ò����õ��IJ����������ձ���©���Ͳ�������
��������ͼ��֪���������X�������������Һ�Ͳ�����ͭ������X������������ˮ������Ӧ�Ļ�ѧ����ʽΪFe+CuSO4=FeSO4+Cu��
�۲�����ͭ����ͭ����������������ҺY�õ�ͭ������������Һ��Y������ͭ��ϡ���ᣬ����������ͭ��Ӧ����ͭ����������������ϡ���ᷴӦ����������������������ͭ�������ᷴӦ��������Һ��������������Һ�����嶼��ͭ��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��Сǿͬѧǰ�����ص�ʯ��ʯ�������е��飬��ȡ�������ɿ�ʯ��ʯ��Ʒ������Ʒ��̼��Ƶ������������м�⣬�������·�����ȡ8g����ʯ��ʯ��Ʒ����40gϡ�����4�μ��룬�������������������±���ʾ(��֪ʯ��ʯ��Ʒ�к������ʲ�����ˮ��Ҳ����ϡ���ᷴӦ)��
��� | ����ϡ���������/g | ʣ����������/g |
��1�� | 10 | 5.5 |
��2�� | 10 | M |
��3�� | 10 | 1.2 |
��4�� | 10 | 1.2 |
����㣺
(1)��Ʒ��̼��Ƶ���������Ϊ________________________________��
(2)����m����ֵӦΪ______________________________��
(3)����ϡ�����������������________________��