��Ŀ����
ʵ��װ����ͼ��ʾ���Թ���װ��ˮ12g�������9g��������Թܵײ����в���δ�ܵĹ��壮�����Թܷ����ձ��и�ˮ���Ȳ�ҡ���Թܣ����ձ��е��¶ȴ�60��ʱ���Թ��еĹ���ǡ����ȫ�ܽ⣮�����Ƽ��ȹ�������������ˮ�������йش��Թ�����Һ����������ȷ���ǣ� ��
A������ǰ����Һ�Ѵﱥ��״̬
B�������¼�����60��Ĺ����У�����Һ��Ũ�Ȳ���
C����60��ʱ����Һ����������Ϊ75%
D����60�������65��ʱ����Һ��������������
���𰸡�������A��������Һ��ָ��һ���¶���һ������ˮ�в��ܼ����ܽ�ij�����ʵ���Һ��
B����Һ��������������= ×100%�����ܼ���������������������ʱ����Һ������������������
×100%�����ܼ���������������������ʱ����Һ������������������
C��������Һ��������������= ×100%������60��ʱ������Һ����������������
×100%������60��ʱ������Һ����������������
D�����ʡ��ܼ���������ʱ���ı���Һ�¶Ȳ���Ӱ����Һ����������������
����⣺A������ǰ�Թܵײ����в���δ�ܵĹ��壬��ʱ��Һ�����ټ����ܽ�����أ����жϼ���ǰ����Һ�Ѵﱥ��״̬����A��ȷ��
B�������¼�����60��Ĺ����У��Թ��еĹ��岻���ܽ⣬��60��ʱǡ����ȫ�ܽ⣻�˹�������������ز����ܽ��ʹ��Һ��������������������B���䣻
C��60��ʱ��9g�������ȫ�ܽ���12gˮ�У����γ���Һ��������������= ×100%��42.9%��75%����C����ȷ��
×100%��42.9%��75%����C����ȷ��
D��60��ʱ��9g�������ȫ�ܽ���12gˮ�У��¶�������65��ʱ����Һ�����ʡ��ܼ����������䣬���жϴ�ʱ��Һ�������������䣻��D��ȷ��
��ѡAD��
��������Һ��������������= ×100%�����ݸù�ʽ����ͨ��������Һ��ɵı仯���ж϶���Һ����������������Ӱ�죮
×100%�����ݸù�ʽ����ͨ��������Һ��ɵı仯���ж϶���Һ����������������Ӱ�죮
B����Һ��������������=
 ×100%�����ܼ���������������������ʱ����Һ������������������
×100%�����ܼ���������������������ʱ����Һ������������������C��������Һ��������������=
 ×100%������60��ʱ������Һ����������������
×100%������60��ʱ������Һ����������������D�����ʡ��ܼ���������ʱ���ı���Һ�¶Ȳ���Ӱ����Һ����������������
����⣺A������ǰ�Թܵײ����в���δ�ܵĹ��壬��ʱ��Һ�����ټ����ܽ�����أ����жϼ���ǰ����Һ�Ѵﱥ��״̬����A��ȷ��
B�������¼�����60��Ĺ����У��Թ��еĹ��岻���ܽ⣬��60��ʱǡ����ȫ�ܽ⣻�˹�������������ز����ܽ��ʹ��Һ��������������������B���䣻
C��60��ʱ��9g�������ȫ�ܽ���12gˮ�У����γ���Һ��������������=
 ×100%��42.9%��75%����C����ȷ��
×100%��42.9%��75%����C����ȷ��D��60��ʱ��9g�������ȫ�ܽ���12gˮ�У��¶�������65��ʱ����Һ�����ʡ��ܼ����������䣬���жϴ�ʱ��Һ�������������䣻��D��ȷ��
��ѡAD��
��������Һ��������������=
 ×100%�����ݸù�ʽ����ͨ��������Һ��ɵı仯���ж϶���Һ����������������Ӱ�죮
×100%�����ݸù�ʽ����ͨ��������Һ��ɵı仯���ж϶���Һ����������������Ӱ�죮 
��ϰ��ϵ�д�
�����Ŀ
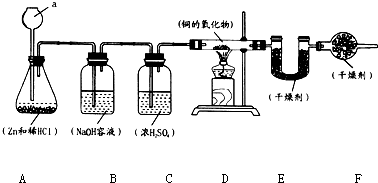 ʵ������п��ϡ������ȡ����������������ԭͭ��һ���������ʵ�����ⶨ�����������ɣ�ʵ��װ����ͼ��ʾ��
ʵ������п��ϡ������ȡ����������������ԭͭ��һ���������ʵ�����ⶨ�����������ɣ�ʵ��װ����ͼ��ʾ�� ��2013?�γ�ģ�⣩ʵ���ҳ��ñ��������������Ȼ����Һ��Ӧ��ȡ�����ĵ�������Ӧ�Ļ�ѧ����ʽΪ��NaNO2+NH4Cl=NaCl+N2+2H2O���˷�Ӧ�Ƿ��ȷ�Ӧ����ʵ��װ����ͼ��ʾ���Իش�
��2013?�γ�ģ�⣩ʵ���ҳ��ñ��������������Ȼ����Һ��Ӧ��ȡ�����ĵ�������Ӧ�Ļ�ѧ����ʽΪ��NaNO2+NH4Cl=NaCl+N2+2H2O���˷�Ӧ�Ƿ��ȷ�Ӧ����ʵ��װ����ͼ��ʾ���Իش� ijͬѧ��Ƶ���һ����̼��ԭ��������ʵ��װ����ͼ��ʾ���Իش��������⣺
ijͬѧ��Ƶ���һ����̼��ԭ��������ʵ��װ����ͼ��ʾ���Իش��������⣺ ��2006?������ijͬѧ��Ƶ���һ����̼��ԭ��������ʵ��װ����ͼ��ʾ��
��2006?������ijͬѧ��Ƶ���һ����̼��ԭ��������ʵ��װ����ͼ��ʾ�� ijͬѧ��Ƶ���һ����̼��ԭ��������ʵ��װ����ͼ��ʾ���Իش��������⣺
ijͬѧ��Ƶ���һ����̼��ԭ��������ʵ��װ����ͼ��ʾ���Իش��������⣺