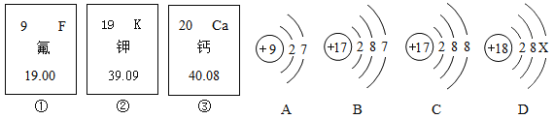
��Ŀ����
����Ŀ��������Ӣ������ѧ�ң��������������ַ����ⶨ�������ܶȡ�
����һ������ȥˮ�����Ͷ�����̼�Ŀ���ͨ���պ��װ��ͭм�IJ����ܣ�ʹ�����е�����ȫ����ȥ����õ������ܶ�Ϊ1.2572kg/L��
����������������NH3��ͨ�����ȵ�װ������ͭ�IJ����ܣ����ɵ�����ˮ��������ȥˮ�������õ������ܶ�Ϊ1.2508g/L��
�����βⶨ��״����ͬ�����ʣ�
��1���������з�����Ӧ�Ļ�ѧ����ʽΪ_____��
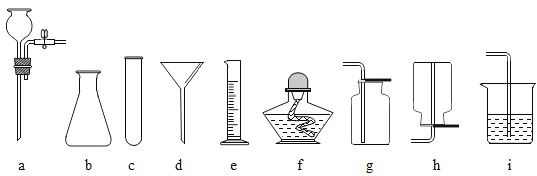
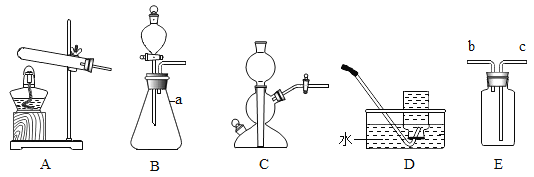
��2������ȥ�����е�ˮ�����Ͷ�����̼���ɹ�ѡ���װ������ͼ��ʾ�������ظ�ʹ�ã�������ѡ���װ���У�����Ӧ����ĸ��ʾ���ܿڵ�����˳��_____��ƿ��Ӧʢ��ʲô����_____��
��3����������ַ������ⶨ�ĵ����ܶ���ֵ��ͬ��ԭ��_____��
���𰸡�2NH3+3CuO![]() 3Cu+N2+3H2O BAGH����GHBA ����������Һ��Ũ���� ����һ�е����庬��ϡ��������������������Ǵ�����
3Cu+N2+3H2O BAGH����GHBA ����������Һ��Ũ���� ����һ�е����庬��ϡ��������������������Ǵ�����
��������
�������е�֪ʶ���з�����������������ͭ��Ӧ����ͭ��������ˮ��Ҫ��ȥ������̼��ˮ����Ҫʹ������������Һ��Ũ���ᣬʹ�ó�ȥ�����ж�����̼��ˮ�����Լ������ķ����ⶨ�������ܶȣ����ں���ϡ�����壬��Խ����һ����Ӱ�졣
��1��������������ͭ��Ӧ����ͭ��������ˮ�Ļ�ѧ����ʽ��2NH3+3CuO![]() 3Cu+N2+3H2O��
3Cu+N2+3H2O��
��2��������ϴ�������Զ�Ӧ�����̳������ܿڵ�����˳��BAGH����GHBA����ȥ�����е�ˮ�����Ͷ�����̼������ʹ������������Һ��Ũ���ᣬ��Ҫ�ȳ�ȥ������̼���������ĸ��
��3��ʹ�ó�ȥ�����ж�����̼��ˮ�����Լ������ķ����ⶨ�������ܶȣ����ں���ϡ�����壬��Խ����һ����Ӱ�죬��ʹ�ð���������ͭ��Ӧ��ȥˮ�ⶨ�ij����������ܶȣ����Է���һ�е����庬��ϡ��������������������Ǵ�������

 �п�������㾫��ϵ�д�
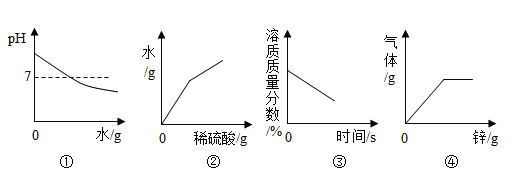
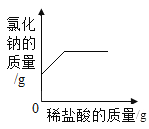
�п�������㾫��ϵ�д�����Ŀ������4������ͼ�ֱ��ʾ4��ʵ�������ijЩ���������ı仯��������ȷ���ǣ���
A | B | C | D |
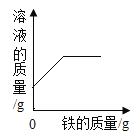
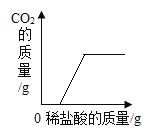
��һ�������������еμӹ���������Һ | ��һ��������ͭ��Һ�в��ϼ������� | ��һ��������ʯ�еμ�ϡ���� | ��һ�����Ȼ��ƺ�̼�����ƻ�����еμ�ϡ���� |
|
|
|
|
A.AB.BC.CD.D