��Ŀ����
����Ŀ����8�֣��˽����ʵ���ɺͽṹ����������ʶ���ʵ����ʡ�
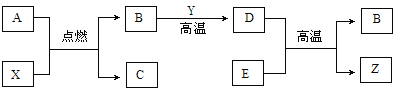
��1����ͼ��ʾ�˵����Ϊ11-17��Ԫ����ߺ���ͻ��ϼۡ�
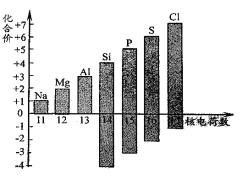
����ͼ�п��Կ�������Ԫ�ص���������� ��
����ijԪ�ؼȿ������ۣ��ֿ��Ը��ۣ����������������۾���ֵ�Ĵ�����Ϊ ��
��д��ͼ��Ԫ�صĻ��ϼ����ź˵����������һ���仯���� ��
��2����3Cu+8HNO3(ϡ)=3Cu(NO3)2+2X��+4H2O��Ӧ�У�
��X�Ļ�ѧʽΪ ��
��HNO3�е�Ԫ�ص���������Ϊ ������һλС������
��Cu(NO3)2��ͭԪ�ء���Ԫ�ص�������Ϊ ��
���÷�Ӧ�漰�������У�ֱ����ԭ�ӹ��ɵ��� ��ˮ���� ���ɡ�
���𰸡���1����+7����8������������������ӣ���2����NO����22.2%����2:3����Cu��ˮ���ӣ�H2O)��
��������
�����������1������ͼ�п��Կ�������Ԫ�ص����������+7��
����ijԪ�ؼȿ������ۣ��ֿ��Ը��ۣ���ͼ�п��Կ������������������۾���ֵ�Ĵ�����Ϊ8��
����ͼ�п��Կ�����ͼ��Ԫ�صĻ��ϼ����ź˵����������һ���仯��������������������ӣ�
��2����3Cu+8HNO3(ϡ)=3Cu(NO3)2+2X��+4H2O��Ӧ�У�
����ѧ��Ӧǰ��ԭ�ӵ�����������䣬X�Ļ�ѧʽΪNO��
��HNO3�е�Ԫ�ص���������Ϊ=![]() ��100%��22.2%��
��100%��22.2%��
��Cu(NO3)2��ͭԪ�ء���Ԫ�ص�������Ϊ=64:(16��3��2)=2:3��
��������ԭ��ֱ�ӹ��ɣ���ֱ����ԭ�ӹ��ɵ���Cu��ˮ����ˮ���ӣ�H2O)���ɡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�