��Ŀ����
�ڻ�ѧʵ��������Կ��ϣ�С��ͬѧ��������������ҩƷ�����������ȡ������ʵ�飮��ش�
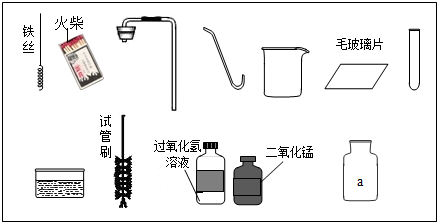
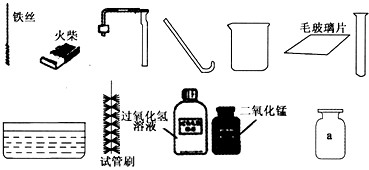
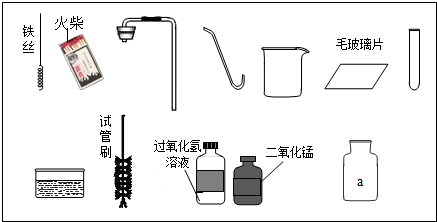
��1��ָ��ͼ������a������______��
��2����ʵ��̨���ṩ��������ҩƷ��С�������Ʊ������ԭ���ǣ��û�ѧ����ʽ��ʾ��______��
��3��������С�����ʵ�����Ҫ����ʾ��ͼ��
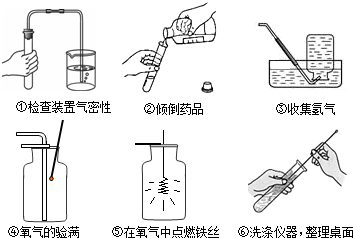
���������ֱ���ÿ�������ȷ��l�֣�����6�֣�ʵ����Ϻ�С������3�֣���ʧ�ֵIJ�����______����д��ţ���˵����ԭ��д������һ��ԭ�ɣ�______��
��4�����ã�2����������ҩƷ��ѡ����С��������ȡ��һ�ֿ��������������ռ��ij������壮���������ķ�����______���û�ѧ����ʽ��ʾ����
��1��ָ��ͼ������a������______��
��2����ʵ��̨���ṩ��������ҩƷ��С�������Ʊ������ԭ���ǣ��û�ѧ����ʽ��ʾ��______��

��3��������С�����ʵ�����Ҫ����ʾ��ͼ��

���������ֱ���ÿ�������ȷ��l�֣�����6�֣�ʵ����Ϻ�С������3�֣���ʧ�ֵIJ�����______����д��ţ���˵����ԭ��д������һ��ԭ�ɣ�______��
��4�����ã�2����������ҩƷ��ѡ����С��������ȡ��һ�ֿ��������������ռ��ij������壮���������ķ�����______���û�ѧ����ʽ��ʾ����
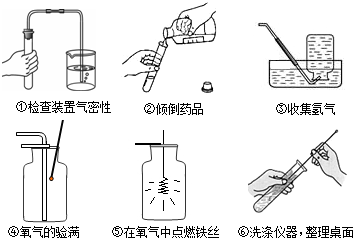
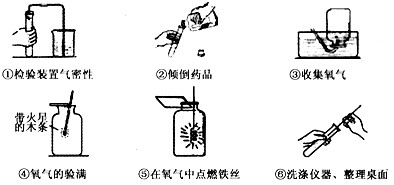
��1�����ռ�������������м���ƿ
��2����ͼ�е�ҩƷ��֪�ǹ���������Һ�ڶ����������������������ȡ����������仯ѧ����ʽ
��3�����Тڴ����㵹Һ��ʱ����ǩû���������Ģܴ�����������ʱ��ľ��û����ƿ�ڢݴ�����˿ȼ�յ�ƿ��û��������ˮ��ϸɳ��ֹƿ��ը��
��4��������̼�ܹ��dz����ʯ��ˮ����ǣ�����̼��ư�ɫ����
����ȷ�𰸣�
��1������ƿ ��2��2H2O2
2H2O+O2��
��3���ڢܢݢ��㵹Һ��ʱ����ǩû���������ģ������������ʱ��ľ��û����ƿ�ڻ����˿ȼ�յ�ƿ��û��������
��4��CO2+Ca��OH��2=CaCO3��+H2O
��2����ͼ�е�ҩƷ��֪�ǹ���������Һ�ڶ����������������������ȡ����������仯ѧ����ʽ
��3�����Тڴ����㵹Һ��ʱ����ǩû���������Ģܴ�����������ʱ��ľ��û����ƿ�ڢݴ�����˿ȼ�յ�ƿ��û��������ˮ��ϸɳ��ֹƿ��ը��
��4��������̼�ܹ��dz����ʯ��ˮ����ǣ�����̼��ư�ɫ����
����ȷ�𰸣�
��1������ƿ ��2��2H2O2
| ||
��3���ڢܢݢ��㵹Һ��ʱ����ǩû���������ģ������������ʱ��ľ��û����ƿ�ڻ����˿ȼ�յ�ƿ��û��������
��4��CO2+Ca��OH��2=CaCO3��+H2O

��ϰ��ϵ�д�
�����Ŀ