��Ŀ����
ij��ѧ��ȤС���ͬѧ���кͷ�Ӧʵ��ʱ����ϡ�����������������Һ�У����⿴�������ݲ��������������������Լ�ƿ�ڷ����а�ɫ��ĩ״���ʣ����Ǵ��һ����Ϊ�����������Ѿ������ˣ��ݴ˻ش��������⣺
��1������������Һ���ʵ�ԭ���� ��
��2������������ʵ�鲻ͬ��ԭ����ͬѧ���������һ��ʵ���ٴ�ȷ�ϸ�����������Һ�Ѿ����ʣ�
��3��������������Һ�Dz��ֱ��ʻ���ȫ�����ʣ�
��4��ʵ����������һ���Ȼ�þ���Ȼ��ƵĹ���������Ʒ��ijͬѧȡ����Ʒ12.8g��ʹ֮��ȫ�ܽ���53gˮ�У��������м���40��20%������������Һ������þ����������ǡ����ȫ��Ӧ����Ӧ����ˣ���12.8����Ʒ���Ȼ�þ��������
��1������������Һ���ʵ�ԭ����
��2������������ʵ�鲻ͬ��ԭ����ͬѧ���������һ��ʵ���ٴ�ȷ�ϸ�����������Һ�Ѿ����ʣ�
| ʵ����� | ʵ������ | ʵ����� |
| ȡ����������������Һ���Թ��У��μ� | ������������Һ�ѱ��� |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| 1ȡ����������������Һ���Թ��� �� 2�������� 3���� 4����Һ�еμ� | 2�а�ɫ�������� 4 | ����������Һ |
���㣺ҩƷ�Ƿ���ʵ�̽��,��Ļ�ѧ����,�εĻ�ѧ����,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺��ѧ̽��
��������1�������������ƻ�������еĶ�����̼��Ӧ���з�����
��2�������������Ʊ��ʺ�IJ���ѡ����ʵ����ʽ��м�����
��3����������������ȫ���������ȫ�����̼���ƣ����Կ��Ը����������ƺ�̼���Ƶ����������ʵ�鷽�����ó����ۣ�
��4���������е����ݽ�ϻ�ѧ����ʽ���м��㣮
��2�������������Ʊ��ʺ�IJ���ѡ����ʵ����ʽ��м�����
��3����������������ȫ���������ȫ�����̼���ƣ����Կ��Ը����������ƺ�̼���Ƶ����������ʵ�鷽�����ó����ۣ�
��4���������е����ݽ�ϻ�ѧ����ʽ���м��㣮
����⣺��1������������Һ�ܹ��Ͷ�����̼��Ӧ����������������Һ����֮��Ҫ����̼���ƣ�
��2������������Һ����֮��IJ���̼���ƣ�̼���ƿ��Ժ��������Ʒ�Ӧ����̼��Ƴ�����������������Һ���ܺ��������Ʒ�Ӧ�����Կ���ѡ������������Һ����֤����������Һ�Ƿ���ʣ�
��3�������������ȫ�����ʣ���ȫ��������̼���ƣ����Կ������ų�̼���Ƹ��ŵ�����������֤�Ƿ����������Ƽ��ɣ����Կ���ѡ������������Ȼ�����Һ����̼����ת��Ϊ������Ȼ�����ⶨ����ɫ��̪���ⶨ��Һ������Լ��ɵó����ۣ�
��4����12.8����Ʒ���Ȼ�þ������Ϊx��
MgCl2+2NaOH=Mg��OH��2+2NaCl
95 80
x 40g��20%
=
x=9.5g��
�ʴ�Ϊ����1��������е�CO2��Ӧ����Na2CO3��
��2��
��3��
��4��9.5g��
��2������������Һ����֮��IJ���̼���ƣ�̼���ƿ��Ժ��������Ʒ�Ӧ����̼��Ƴ�����������������Һ���ܺ��������Ʒ�Ӧ�����Կ���ѡ������������Һ����֤����������Һ�Ƿ���ʣ�
��3�������������ȫ�����ʣ���ȫ��������̼���ƣ����Կ������ų�̼���Ƹ��ŵ�����������֤�Ƿ����������Ƽ��ɣ����Կ���ѡ������������Ȼ�����Һ����̼����ת��Ϊ������Ȼ�����ⶨ����ɫ��̪���ⶨ��Һ������Լ��ɵó����ۣ�
��4����12.8����Ʒ���Ȼ�þ������Ϊx��
MgCl2+2NaOH=Mg��OH��2+2NaCl
95 80
x 40g��20%
| 95 |
| x |
| 80 |
| 40g��20% |
x=9.5g��
�ʴ�Ϊ����1��������е�CO2��Ӧ����Na2CO3��
��2��
| ʵ����� | ʵ������ | ʵ����� |
| ȡ����������������Һ���Թ��У��μ� Ca��OH��2��Һ | ���ɰ�ɫ���� | ������������Һ�ѱ��� |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| 1ȡ����������������Һ���Թ��У� 2��������CaCl2��Һ�� 3���� 4����Һ�еμ���ɫ��̪��Һ | 2�а�ɫ�������� 4��Һ��� | ������������Һ���ֱ��� |
��������������̼���ƺ��������Ƶ����ʣ���������������ǣ�������ʱ����̼������Һ�ʼ�����ʹ��ɫ��̪��죬�����ڼ�������������̼���ƻ����Һ�е���������ʱ���������Ȼ��ƻ��Ȼ����Ȱ�̼���Ʒ�Ӧ������ȥ���ٵμӷ�̪�ķ������м��飮

��ϰ��ϵ�д�
�����Ŀ
���л�ѧ����ʽ��д��ȷ���ǣ�������
| A��2Fe+6HCl=2FeCl3+3H2�� | ||||
| B��2H2O=O2��+2H2�� | ||||
C��CO2+C
| ||||
| D��SO2+NaOH=Na2SO3+H2O |
 ��ѧϰ�ꡰ������̼���ʡ���ij�ѧ��ȤС���ͬѧ���ѩ�����ݳ����������̽������ͼ��ѩ����ˮ���ϱ���
��ѧϰ�ꡰ������̼���ʡ���ij�ѧ��ȤС���ͬѧ���ѩ�����ݳ����������̽������ͼ��ѩ����ˮ���ϱ���
 ��ͼ��ʾ��Ϊ�ⶨ������Һ���������Ƶĺ�����С��ȡ25mL���˷�̪��Ұ�ķ�Һ����ƿ�У���������μ���������������Ϊ7.3%��ϡ���ᣬ����Һ��ɫǡ�ñ�Ϊ��ɫʱ���ζ�����ʣ��ϡ����Ϊ15mL�����÷�Һ��ϡ�����ܶȾ�ԼΪ1g/cm3����������Һ���������Ƶ���������������
��ͼ��ʾ��Ϊ�ⶨ������Һ���������Ƶĺ�����С��ȡ25mL���˷�̪��Ұ�ķ�Һ����ƿ�У���������μ���������������Ϊ7.3%��ϡ���ᣬ����Һ��ɫǡ�ñ�Ϊ��ɫʱ���ζ�����ʣ��ϡ����Ϊ15mL�����÷�Һ��ϡ�����ܶȾ�ԼΪ1g/cm3����������Һ���������Ƶ���������������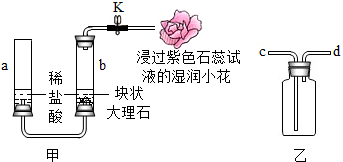 ijʵ��С������ͼװ���о�CO2�����ʣ�
ijʵ��С������ͼװ���о�CO2�����ʣ� �ס��������ʵ��ܽ��������ͼ��ʾ�����ͼ�ش��������⣮
�ס��������ʵ��ܽ��������ͼ��ʾ�����ͼ�ش��������⣮ ��ͼ��ijͬѧ�����ľ�ˮ������ش��������⣺
��ͼ��ijͬѧ�����ľ�ˮ������ش��������⣺