��Ŀ����
��3�֣���������ƣ�Na2S2O3����һ����;�㷺�����ʡ�ij�����������Ʒ�к��������������ơ���ȡ16 g����Ʒ�����ձ��У�����113.6 gһ����������������ϡ����ǡ����ȫ��Ӧ���õ�120 g�����Ʋ�������Һ��
������Ӧ�Ļ�ѧ����ʽΪ��Na2S2O3 + H2SO4=== Na2SO4 + H2O + S��+ SO2��
����㣺
��1����Ʒ����������ƣ�Na2S2O3���������Ƶ������ȡ�
��2��������Һ����������������
������Ӧ�Ļ�ѧ����ʽΪ��Na2S2O3 + H2SO4=== Na2SO4 + H2O + S��+ SO2��
����㣺
��1����Ʒ����������ƣ�Na2S2O3���������Ƶ������ȡ�
��2��������Һ����������������
��1��79��1 ��2��12%
�����������1�����������غ㶨�ɿ����жϣ�����S��SO2��������=16g+113.6g-120g=9.6g���ٸ��ݻ�ѧ����ʽ��Na2S2O3 + H2SO4=== Na2SO4 + H2O + S��+ SO2����S��SO2��������=1��2������S������Ϊx����SO2������Ϊ2x������X+2x=9.6g��x=3.2g
�⣺ ���������������Ϊy�����ɵ�����������Ϊz
Na2S2O3 + H2SO4=== Na2SO4 + H2O + S��+ SO2��
158 142 32 64
y z 3.2 g
y="15.8" g z="14.2" g
��1����Ʒ������������������Ƶ�������Ϊ��15.8 g : (16 g - 15.8 g) = 79:1
��2����������Һ��������������
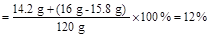
�𣺣�1����Ʒ������������������Ƶ�������Ϊ79:1��
��2����������Һ��������������Ϊ12%��

��ϰ��ϵ�д�
�����Ŀ

 C6H12O7+Cu2O��+2H2O
C6H12O7+Cu2O��+2H2O