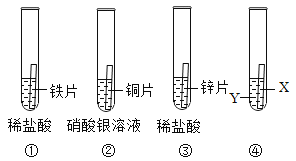
��Ŀ����
����Ŀ�����ʵ���ɺͽṹ�ǻ�ѧ�о�����Ҫ���ݡ�
��1����ͼ�Dz���Ԫ�ص�ԭ�ӻ����ӽṹʾ��ͼ
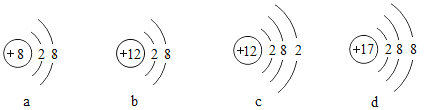
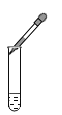
�ٱ�ʾ�����ӵĽṹʾ��ͼ��_______������a������b������c������d������ͬ���������ȶ��ṹ������______��
��_______����ͬ��Ԫ�ص�����
��2��![]() ��ʾ̼ԭ�ӣ�
��ʾ̼ԭ�ӣ�![]() ��ʾ��ԭ��,�����ܱ�ʾ���ָɱ��Ļ�ѧ���ʵ�����_______��
��ʾ��ԭ��,�����ܱ�ʾ���ָɱ��Ļ�ѧ���ʵ�����_______��
A![]() B
B![]() C
C![]() D
D![]()
��3����Ԫ�أ�Nd�������쵼���Ľ��������е���Ҫ����Ԫ�أ���Ԫ�ز�����Ϣ��ͼ��ʾ��
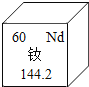
����Ԫ�ص����ԭ������Ϊ____����Ԫ������____�����������������ǽ�������Ԫ�أ�
�ڸ�Ԫ��ԭ�ӵĺ˵����Ϊ____��
����֪�Ȼ��ϵĻ�ѧʽΪNdCl3���������ϵĻ�ѧʽΪ____��
��4���ؿ��к������Ľ���Ԫ�ء��ǽ���Ԫ�غͿ����к�������Ԫ����ɵĻ����ﻯѧʽ��____��
A Al(NO3)3 B CaCO3 C Al2(SO4)3 D Fe(NO3)3
��5���ҹ����������������Ĵ���������������ʢ����������ĸ���������Ҫ�ɷֵĻ�ѧʽΪ��Al2(Si2O5) (OH)4���������մ���������ԭ�ϡ��������й����Ԫ�صĵ�������Ϊ____��
���𰸡�b abd bc D 144.2 ���� 60 Nd2O3 A 7:18
��������
��������=�����������Ϊԭ�ӣ���������>�����������Ϊ�����ӣ���������<�����������Ϊ�����ӣ���ԭ�ӵ�����������Ϊ8����Ϊ2��������������ȶ��ṹ��ͬ��Ԫ�ص���������ͬ��
(1)���������У�b��������=12�����������=10��������>�����������Ϊ�����ӣ�abd��������������8�������ȶ��ṹ�����������У�bc������������������ͬ������ͬһ��Ԫ�ء�
(2)�ɱ����ɶ�����̼���ӹ��ɵģ����ָɱ��Ļ�ѧ���ʵ����Ļ�ѧ���ʵ����Ƕ�����̼���ӣ���ѡD��
(3)����Ԫ�ز�����Ϣͼ2��֪������Ԫ�ص����ԭ������Ϊ144.2����Ԫ�ص������д��н����ԣ����ڽ���Ԫ�أ��ڸ�Ԫ��ԭ�ӵĺ˵����Ϊ60��
����֪�Ȼ��ϵĻ�ѧʽΪ![]() ,����Ԫ�صĻ��ϼ�Ϊ+3��,��Ԫ�صĻ��ϼ�Ϊ2��,�������ϵĻ�ѧʽΪ
,����Ԫ�صĻ��ϼ�Ϊ+3��,��Ԫ�صĻ��ϼ�Ϊ2��,�������ϵĻ�ѧʽΪ![]() ��
��
(4)�ؿ��к������Ľ���Ԫ������Ԫ�ء��ǽ���Ԫ������Ԫ��,�����к�������Ԫ���ǵ�Ԫ�أ���ɵĻ�����������������ѡA��
(5)�������й����Ԫ�ص�������Ϊ![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�