��Ŀ����
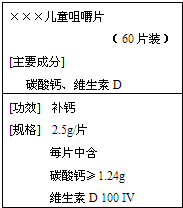 ��2008?��������ģ����ͼ�ǡ���������������Ʒ��ǩͼ�����ݱ�ǩ��Ϣ�ش�
��2008?��������ģ����ͼ�ǡ���������������Ʒ��ǩͼ�����ݱ�ǩ��Ϣ�ش���1����Ҫ�ɷ�̼��Ƶ�ʽ��
100
100
����2��ijͬѧΪ�ⶨ̼��Ƶĺ�����ע�Ƿ���ʵ��ȡ��4ƬƬ�������������ձ�����μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥϡ����40g�������ձ���ʣ����������Ϊ47.8g�������ձ��������ٶ���Ƭ�����ɷֲ������ᷴӦ����
����
�����ɶ�����̼��������
��ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��
��������1������̼��ƵĻ�ѧʽ����ʽ����
��2���ٸ��������غ㶨�ɽ��з������ձ��е����ʼ��ٵ������������ɵĶ�����̼�����������ɶ�����̼�����������ݷ�Ӧ�ķ���ʽ�����ÿƬ��̼��Ƶ������������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��
��2���ٸ��������غ㶨�ɽ��з������ձ��е����ʼ��ٵ������������ɵĶ�����̼�����������ɶ�����̼�����������ݷ�Ӧ�ķ���ʽ�����ÿƬ��̼��Ƶ������������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��
����⣺��1��̼��ƵĻ�ѧʽ��CaCO3��ʽ��Ϊ��40+12+16��3=100��
��2������Ϊ̼��ƺ�ϡ���ᷴӦ�ų�������̼�����������غ㶨�ɿ�֪���ձ��е����ʼ��ٵ������������ɵĶ�����̼�����������ԣ����ɶ�����̼������Ϊ��2.5g��4+40g-47.8g=2.2g��
����4ƬƬ����̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 2.2g
=
x=5g
ÿƬ�к���̼��Ƶ�����Ϊ��
=1.25g��1.24g
���ԣ���Ƭ��̼��Ƶĺ�����ע��ʵ��
�ʴ�Ϊ����1��100����2�������ɶ�����̼������Ϊ2.2g���ڸ�Ƭ��̼��Ƶĺ�����ע����ʵ��
��2������Ϊ̼��ƺ�ϡ���ᷴӦ�ų�������̼�����������غ㶨�ɿ�֪���ձ��е����ʼ��ٵ������������ɵĶ�����̼�����������ԣ����ɶ�����̼������Ϊ��2.5g��4+40g-47.8g=2.2g��
����4ƬƬ����̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 2.2g
| 100 |
| 44 |
| x |
| 2.2g |
ÿƬ�к���̼��Ƶ�����Ϊ��
| 5g |
| 4 |
���ԣ���Ƭ��̼��Ƶĺ�����ע��ʵ��
�ʴ�Ϊ����1��100����2�������ɶ�����̼������Ϊ2.2g���ڸ�Ƭ��̼��Ƶĺ�����ע����ʵ��
�������������������غ㶨�������Ӧ�ų�������̼���������ǽ��к������Ļ��������ֳ�����֪ʶ���������������

��ϰ��ϵ�д�
�����Ŀ