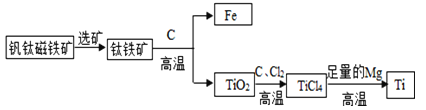
��Ŀ����
����Ŀ��A-E�dz��л�ѧ�еij������ʣ���һ���������ܷ�������ת������֪A,B��Ϊ��ɫ���壬C,EΪ���壬��E��������ѪҺ��Ѫ�쵰��ϣ�ʹ���ж���
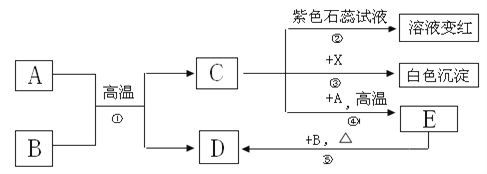
��ش��������⣺
��1��A,B���������������_________����A��B��������X�Ļ�ѧ����Ϊ_______��
��2����Ӧ�ٵĻ�ѧ����ʽΪ___________________________________________��
��Ӧ�ܵĻ�ѧ����ʽΪ___________________________________________��
���ȷ�Ӧ��������Һ���ɹ۲쵽��������___________________________��
��ѧ����ʽΪ___________________________________________________��
E�ڷ�Ӧ���б��ֳ��Ŀ�����ұ��ҵ ����Ҫ��ѧ������____________��
���𰸡� B �������ƣ� C+2CuO![]() 2Cu+CO2�� CO2+C
2Cu+CO2�� CO2+C![]() 2CO ��ɫ��Һ�ֱ����ɫ H2CO3 = CO2+H2O ��ԭ��
2CO ��ɫ��Һ�ֱ����ɫ H2CO3 = CO2+H2O ��ԭ��
����������1��E��������ѪҺ��Ѫ�쵰��ϣ�ʹ���ж�����E��һ����̼��C������ʯ���죬C��A��Ӧ������һ����̼����C�Ƕ�����̼��A��̼����A��B�����ɶ�����̼��D����B�Ǻ�ɫ���壬��A������ͭ��D��ͭ����AB�������������������ͭ��ѡB��X���������̼��Ӧ���ɰ�ɫ��������X���������ƣ�
��2��̼������ͭ�ڸ��µ������·�Ӧ����ͭ�Ͷ�����̼����Ӧ����ʽΪC+2CuO![]() 2Cu+CO2����������̼��̼�ڸ��µ������·�Ӧ����һ����̼����Ӧ����ʽΪCO2+C
2Cu+CO2����������̼��̼�ڸ��µ������·�Ӧ����һ����̼����Ӧ����ʽΪCO2+C![]() 2CO��̼��ȶ��������ֽ⣬��ʯ���к�ɫ��Ϊ��ɫ��̼��ֽ����ɶ�����̼��ˮ����Ӧ����ʽΪH2CO3 = CO2+H2O��E��һ����̼�������仹ԭ�ԣ�����ұ��������
2CO��̼��ȶ��������ֽ⣬��ʯ���к�ɫ��Ϊ��ɫ��̼��ֽ����ɶ�����̼��ˮ����Ӧ����ʽΪH2CO3 = CO2+H2O��E��һ����̼�������仹ԭ�ԣ�����ұ��������

 ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�