��Ŀ����
�ձ���װ��һ�������������ͭ�Ļ����Һ���������Һ�м���������BaCl2��Һ������46.6�˳������������Һ�м���10%��
NaOH��Һ���õ�������������¼���£�
��1���û����Һ��CuSO4������Ϊ______�ˣ�
��2������NaOH��Һ______��ʱ���õ��������������ٱ仯��
NaOH��Һ���õ�������������¼���£�
| ����NaOH��Һ������/g | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
| ����������������/g | 0.0 | 2.5 | 8.6 | 9.8 | 9.8 |
��2������NaOH��Һ______��ʱ���õ��������������ٱ仯��
��1���ɼ�¼���ݱ���֪��������ͭ��ȫ��Ӧ��������ɫ����9.8g��
����Һ������ͭ������Ϊy
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
160 98
y 9.8g
=
y=16g
�ʴ�Ϊ��16
��2����μ�150gNaOH��Һ֮����ʹ����ȫ��������μ�����������Һ������Ϊx
=
x=10g
�������������ٱ仯ʱ����NaOH��Һ������Ϊ��150g+10g=160g
�ʴ�Ϊ��160
����Һ������ͭ������Ϊy
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
160 98
y 9.8g
| 160 |
| y |
| 98 |
| 9.8g |
y=16g
�ʴ�Ϊ��16
��2����μ�150gNaOH��Һ֮����ʹ����ȫ��������μ�����������Һ������Ϊx
| 150g-100g |
| 8.6g-2.5g |
| x |
| 9.8g-8.6g |
x=10g
�������������ٱ仯ʱ����NaOH��Һ������Ϊ��150g+10g=160g
�ʴ�Ϊ��160

��ϰ��ϵ�д�
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
�����Ŀ
�ձ���װ��һ�������������ͭ�Ļ����Һ����֪����Һ�к�H2SO4������Ϊ9.8g��ijͬѧΪ�ⶨ�û����Һ������ͭ�����������ձ�������10%��NaOH��Һ���õ�������������¼���£�
��1���õ�������������Ϊ g���û����Һ������ͭ������Ϊ g��
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ���Ҫ��д��������̣�
��3�������ڸû����Һ�м���NaOH��Һ���������ɳ��������仯��ϵ�����ߣ����������
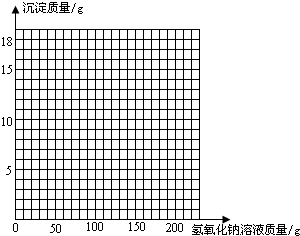
| ����NaOH��Һ������/g | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
| ���ɳ���������/g | 0.0 | 2.5 | 8.6 | 9.8 | 9.8 |
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ���Ҫ��д��������̣�
��3�������ڸû����Һ�м���NaOH��Һ���������ɳ��������仯��ϵ�����ߣ����������

�ձ���װ��һ�������������ͭ�Ļ����Һ����֪����Һ�к�H2SO4������Ϊ9.8g��ijͬѧΪ�ⶨ�û����Һ������ͭ�����������ձ�������10%��NaOH��Һ���õ�������������¼���£�
��1���õ�������������Ϊ g���û����Һ������ͭ������Ϊ g��
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ���Ҫ��д��������̣�
| ����NaOH��Һ������/g | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
| ���ɳ���������/g | 0.0 | 2.45 | 8.6 | 9.8 | 9.8 |
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ���Ҫ��д��������̣�