��Ŀ����
������NH3����һ����ɫ���д̼�����ζ�����壬�ܶ�С�ڿ�������������ˮ������ˮ��Һ��Ϊ��ˮ���Լ��ԣ����ڻ�ѧ��ҵ����;�ܹ㷺�������ƻ��ʡ��ƴ���ȣ����������������ڻ���������
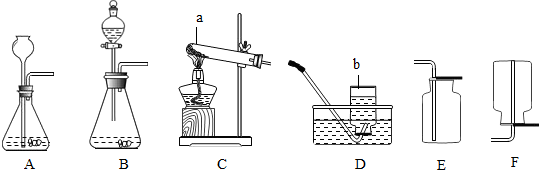
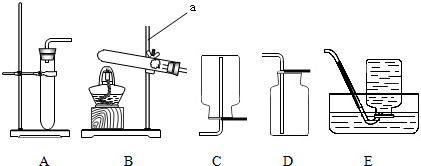
��1��ʵ�����ռ������ɲ��õķ���Ϊ______��
��2��������һ�������£����¡���������������Ӧ����һ��������ˮ����д���÷�Ӧ�Ļ�ѧ����ʽ��______��
��3����400�����ң��д������ڵ������£��ð����ɽ��ж�����NO2ת�����÷�Ӧ�Ļ�ѧ����ʽΪ8NH3+6NO2�T7N2+12A������A�Ļ�ѧʽΪ______��
��4����ҵ�ϣ���ϸ���������ð��������״��ķ�ˮ��ʹ���Ϊ����N2��CO2���Ӷ�����Ի�������Ⱦ���йصķ�ӦΪ��6NH3+5CH3OH+12O2
3N2��+5CO2��+19H2O��������Ӧǰ��NԪ�صĻ��ϼ۵ı仯���Ϊ______ ������ߡ������͡������䡱����
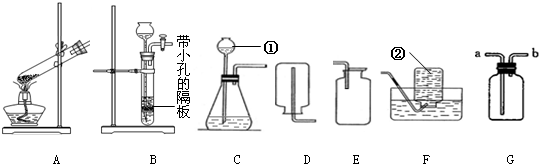
��1��ʵ�����ռ������ɲ��õķ���Ϊ______��
��2��������һ�������£����¡���������������Ӧ����һ��������ˮ����д���÷�Ӧ�Ļ�ѧ����ʽ��______��
��3����400�����ң��д������ڵ������£��ð����ɽ��ж�����NO2ת�����÷�Ӧ�Ļ�ѧ����ʽΪ8NH3+6NO2�T7N2+12A������A�Ļ�ѧʽΪ______��
��4����ҵ�ϣ���ϸ���������ð��������״��ķ�ˮ��ʹ���Ϊ����N2��CO2���Ӷ�����Ի�������Ⱦ���йصķ�ӦΪ��6NH3+5CH3OH+12O2
| ||
��1�������ܶ�С�ڿ��������������ſ������ռ�����������ˮ��������ˮ���ռ���
��2����Ӧ���ǰ�������������������һ��������ˮ������С����������ƽ����Ӧ�����Ǹ��¡�����д�ڵȺŵ����£�
��3����Ӧ������14��N��24��H��12��O��������������14��N������24��H��12��O����ΪA��ǰ��ϵ����12�����Ի�ѧʽ��H2O����
��4�������е��Ļ��ϼ���-3�ۣ������ﵪ���е���0�ۣ����Ի��ϼ����ߣ�
�ʴ�Ϊ����1�������ſ���������2��4NH3+5O2
4NO+6H2O����3��H2O����4�����ߣ�
��2����Ӧ���ǰ�������������������һ��������ˮ������С����������ƽ����Ӧ�����Ǹ��¡�����д�ڵȺŵ����£�
��3����Ӧ������14��N��24��H��12��O��������������14��N������24��H��12��O����ΪA��ǰ��ϵ����12�����Ի�ѧʽ��H2O����
��4�������е��Ļ��ϼ���-3�ۣ������ﵪ���е���0�ۣ����Ի��ϼ����ߣ�
�ʴ�Ϊ����1�������ſ���������2��4NH3+5O2
| ||
| ���� |

��ϰ��ϵ�д�
�����Ŀ