��Ŀ����
����Ŀ��ʵ������������þ�������ƵĹ���������Ʒ��С��ͬѧ��ⶨ��Ʒ������þ�������������ȳ�ȡ�û������Ʒ20g����ȫ����100gˮ�С�Ȼ��ȡ����һ��������������������������Һ100gƽ�����Ĵμ������У������ʵ���������ݼ��±�����������������йؼ��㣺
���� | 1 | 2 | 3 | 4 |
��������������Һ������/g | 25 | 25 | 25 | 25 |
���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
��1���ϱ���X����ֵΪ______��ԭ����������Ʒ������þ������������ ��
��2����ǡ����ȫ��Ӧʱ����Һ�����ʵ������������������ȷ��0.1%��
��3��������ͼ�л�����20 g ��Ʒ�м�����������Һ��������������������仯��ϵ��ʾ��ͼ��
��4����������������Һ��������������Һ���ⶨ��Ʒ������þ�������������Ƿ���У���˵�����ɡ�
���𰸡���1��5.8��90% ����2��12.5%����3��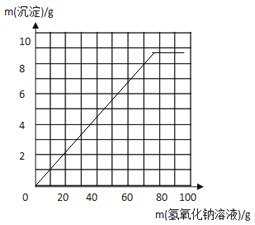 ����4�� ��������Ҳ�������������Ʒ�Ӧ�����²ⶨ���ƫ��
����4�� ��������Ҳ�������������Ʒ�Ӧ�����²ⶨ���ƫ��
����������1���ɱ������ݷ�����֪��ÿ25������������Һ��ȫ��Ӧ������2.9g������þ����������X��ֵΪ5.8�������������Ʒ������þ������Ϊx�����������Ƶ�����Ϊy��
MgSO4 ��2NaOH==Na2SO4��Mg(OH)2��
120 142 58
x y 8.7g
120/x=142/y=58/8.7g
x=21.3g y=18g
ԭ����������Ʒ������þ������������18g��20g��100%=90%;
ǡ����ȫ��Ӧʱ����Һ�����ʵ���������=![]() 12.5%��
12.5%��
��3�� ����4�� ��������Ҳ�������������Ʒ�Ӧ�����²ⶨ���ƫ��
����4�� ��������Ҳ�������������Ʒ�Ӧ�����²ⶨ���ƫ��

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�