��Ŀ����
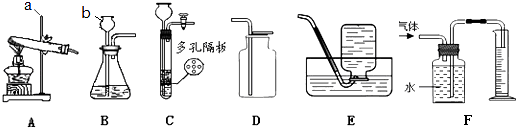
����Ŀ��ij��ѧ��ȤС�������һ�顰������ʵ�飬����װ����ͼ��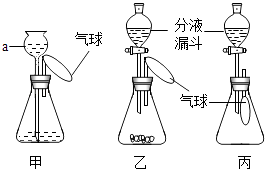
��1����װ�ã�
������a������Ϊ
����a�м�ˮ�ῴ�������ʹ�һ��ʱ���������Сû�б仯��˵����װ�������� ��
��2����װ�ã�
��������O2ʹ�����ʹ�����ƿ����װ�Ĺ������ʿ����� ��
��������H2ʹ�����ʹ���Ӧ�Ļ�ѧ����ʽΪ ��
������ƿ��װ��NaOH���壬��Һ©���м�������ˮ���������ʹ����Ҫԭ���� ��
��3����װ�ã�
����ƿ��ʢ��CO2 �� ��ʹ�����ʹ����Һ©���е�Һ������� �� ��ƿ�з�����Ӧ�Ļ�ѧ����ʽ�� ��
���𰸡�
��1������©��,����
��2����������,Zn+H2SO4=ZnSO4+H2��,NaOH��������ˮ����,������������
��3��NaOH��Һ,2NaOH+CO2=Na2CO3+H2O��
����������1��������a������Ϊ����©��������a�м�ˮ�ῴ�������ʹ�һ��ʱ���ڣ�2���ٸ�װ���ǹ�Һ�ڳ�������ȡ�����װ�ã�������O2ʹ�����ʹ�����ƿ����װ�Ĺ������ʿ����Ǵ����������̣������Һ���ǹ���������Һ����п��ϡ���ᷴӦ��������п����������Ӧ�Ļ�ѧ����ʽΪZn+H2SO4=ZnSO4+H2������������������ˮ�ų��������ȣ�װ������ѹ���������ʹ�3������CO2���屻����ʱ��װ����ѹǿ��С���ڲ�������ͻ��ʹ�����������Һ�����ն�����̼��������̼���ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��2NaOH+CO2=Na2CO3+H2O��
�ʴ�Ϊ������©�������ã��������̣�Zn+H2SO4=ZnSO4+H2����NaOH��������ˮ���ȣ������������ͣ�NaOH��Һ��2NaOH+CO2=Na2CO3+H2O��
������Ҫ����������Ʊ������Ʊ�����֮ǰ����Ҫ���װ�������ԡ�

 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�