��Ŀ����
����Ŀ��ijͬѧ�ڰ�����ʦ����ʵ����ʱ��������һƿ��ǩ��ȱ������������Һ������ͨ��ʵ��֪����ƿ��Һ�����ʵ�������������������ʦͬ����������кͷ�Ӧ�����ʵ�顣ȡ50g����������Һ�����ձ��У�����3����ɫ��̪��Һ��������5%��ϡ���ᣬ���ò��������Ͻ��裬���ձ���Һ��ǡ�ñ�Ϊ��ɫʱ��ȥϡ����100g��(����������һλС��)
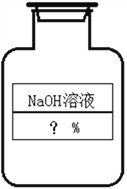
(1)��ƿ����������Һ��������������Ϊ____________��
(2)��Ҫ���Ƹ�������������������������Һ200g,��Ҫ�������ƹ����������________g��
���𰸡� 8.2% 16.4
���������⣺��1�����������ʵ�����Ϊ��5%��100g=5g
���������Ƶ�����Ϊx
2NaOH+H2SO4�TNa2SO4+2H2O
80 98
x 5g
![]()
x��4.1g
��ƿ����������Һ��������������Ϊ![]() ��100%=8.2%
��100%=8.2%
��2����Ҫ�������ƹ����������200g��8.2%=16.4g��
�𣺣�1����ƿ����������Һ��������������Ϊ8.2%��
��2����Ҫ�������ƹ����������16.4g��

��ϰ��ϵ�д�
�����Ŀ