题目内容
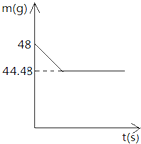 小强同学前往当地的石灰石矿区进行调查,他取回了若干块矿石样品,对样品中碳酸钙的质量分数进行检测,采用的办法如下:取用8g这种石灰石样品,把40g稀盐酸充分反应(已知石灰石样品中含的杂质不溶于水,不与盐酸反应),测得烧杯中的反应剩余物的质量(m)与反应时间(t)的关系如图所示.试计算:
小强同学前往当地的石灰石矿区进行调查,他取回了若干块矿石样品,对样品中碳酸钙的质量分数进行检测,采用的办法如下:取用8g这种石灰石样品,把40g稀盐酸充分反应(已知石灰石样品中含的杂质不溶于水,不与盐酸反应),测得烧杯中的反应剩余物的质量(m)与反应时间(t)的关系如图所示.试计算:(1)生成的二氧化碳的质量为
3.52
3.52
g(2)样品中碳酸钙的质量分数是多少?(写出计算过程)
分析:(1)依据图象中烧杯中的物质的质量之差即为二氧化碳的质量;
(2)依据二氧化碳的质量和反应方程式即可求出碳酸钙的质量,进而得到样品中碳酸钙的质量分数;
(2)依据二氧化碳的质量和反应方程式即可求出碳酸钙的质量,进而得到样品中碳酸钙的质量分数;
解答:解:根据质量守恒定律,可得出反应生成的CO2质量为:48g-44.48g=3.52g
设参加反应的碳酸钙质量为x.
CaCO3+2HCl=CaCl2+H2O+CO2↑
100 44
x 3.52g
=
x=8g
样品中CaCO3 的质量分数=
×100%=100%
故答案为:(1)3.52;(2)答:样品中碳酸钙的质量分数100%.
设参加反应的碳酸钙质量为x.
CaCO3+2HCl=CaCl2+H2O+CO2↑
100 44
x 3.52g
| 100 |
| x |
| 44 |
| 3.52g |
x=8g
样品中CaCO3 的质量分数=
| 8g |
| 8g |
故答案为:(1)3.52;(2)答:样品中碳酸钙的质量分数100%.
点评:在对数形结合类问题中的表示变化的曲线进行分析时,曲线的折点的特殊意义是分析的重点.

练习册系列答案
相关题目
小强同学前往当地的石灰石矿区进行调查,他取回了若干块矿石样品,对样品中碳酸钙的质量分数进行检测,采用了一下方法:取用8g这种石灰石样品,把40g稀盐酸分四次加入,测量过程所得数据见下表(已知石灰石样品中含有的杂质不溶于水,不与盐酸反应).请计算:
(1)8g的石灰石样品中含有杂质 克.
(2)上表中m的数值是 .
(3)样品中碳酸钙的质量分数是多少?
(4)要得到280kg的CaO,需要质量分数为80%的石灰石多少千克?(提示:化学方程式为 CaCO3
CaO+CO2↑)
| 序号 | 加入稀盐酸质量(g) | 剩余固体质量(g) |
| 第1次 | 10 | 5.5 |
| 第2次 | 10 | m |
| 第3次 | 10 | 1.2 |
| 第4次 | 10 | 1.2 |
(2)上表中m的数值是
(3)样品中碳酸钙的质量分数是多少?
(4)要得到280kg的CaO,需要质量分数为80%的石灰石多少千克?(提示:化学方程式为 CaCO3
| ||
小强同学前往当地的石灰石矿区进行调查.他取回了若干块矿石样品,对样品中的碳酸钙质量进行检测.采取了以下办法,取用8克这种石灰石样品,把40克稀盐酸分四次加入,测得过程所得数见下表(已知石灰石样品的含有的杂质不溶于水,也不与盐酸反应)根据数据,请计算:
(1)上表中M 的数值应为______
(2)样品中碳酸钙的质量分数是______
(3)小强所用稀盐酸的溶质质量分数是______.
| 序号 | 第一次 | 第二次 | 第三次 | 第四次 |
| 加入稀盐酸质(克) | 10 | 10 | 10 | 10 |
| 剩余固体质量(克) | 5.5 | M | 1.2 | 1.2 |
(2)样品中碳酸钙的质量分数是______
(3)小强所用稀盐酸的溶质质量分数是______.