��Ŀ����
���ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ʯ��ʯ��Ʒ�е��������Ȳ��ֽ⣬�Ҳ����ᷴӦ�����ס�����λͬѧ�ֱ��������������ʵ�鷽����
����һ���ٳ�ȡʯ��ʯ��Ʒ����8g�����þƾ��Ƽ�����Ʒ��ֱ���������ٸı䣻�۽������������ڸ������������ȴ��Ƶ�����6.9 g���ܼ��㣮
���������ٳ�ȡʯ��ʯ��Ʒ����8 g���ڼ�����������Ϊ7.3%������100 g��ʹ̼�����ȫ��Ӧ������Ӧ�����Һ�м��뺬����3.2 g������������Һ��ǡ���кͶ��������ܼ��㣮
��ش������й����⣺
��1��100g��������Ϊ7.3%�������У������Ȼ��������Ϊ______g��
��2������Ϊ���������У���ʵ���е��Ƿ���______����ѡ����һ������ԭ����______��
��3��������Ʒ��̼��Ƶ�����������
�⣺��1��100g��7.3%=7.3g���ʴ�Ϊ��7.3g��
��2���������������Կ�������һ�������Ģ����þƾ��Ƽ��ȣ��¶ȴﲻ��ʯ��ʯ�ֽ�ĸ���Ҫ��������̼��ƿ��Ժ����ᷢ����Ӧ��
�ʴ�Ϊ������̼���Ҫ�ڸ����²�����ȫ�ֽ⣮
��3���⣺�������������Ʒ�Ӧ��HCl����Ϊx
HCl+NaOH�TNaCl+H2O
36.5 40
x 3.2g
��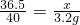
��֮�ã�x=2.92g��
��ô����̼��Ʒ�Ӧ����������Ϊ��7.3%��100g-2.92g=4.38g��
����ʯ��ʯ��̼��Ƶ�����Ϊy��
CaCO3+2HCl=CaCl2+CO2��+H2O
100 73
y 4.38g
��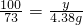
��֮��y=6g��
̼��Ƶ���������Ϊ��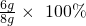 =75%��
=75%��
�𣺷�������Ʒ��̼��Ƶ���������Ϊ75%��
��������1��������������=��Һ���������������������㼴�ɣ�
��2���������������Կ�������һ�������Ģ����þƾ��Ƽ��ȣ��¶ȴﲻ��ʯ��ʯ�ֽ�ĸ���Ҫ��������̼��ƿ��Ժ����ᷢ����Ӧ��
��3���ٸ�������������Һ�����ᷴӦ�Ļ�ѧ����ʽ��������������Ʒ�Ӧ��HCl������Ȼ�����������HCl������-�������Ʒ�Ӧ��HCl����=��̼��Ʒ�Ӧ��HCl��������
�ڸ���̼��ƺ����ᷴӦ�Ļ�ѧ����ʽ�����̼��Ƶ��������ٸ�������������ʽ���㼴�ɣ�
������������Ҫ����ѧ�����û�ѧ����ʽ������������ʽ���м����������
��2���������������Կ�������һ�������Ģ����þƾ��Ƽ��ȣ��¶ȴﲻ��ʯ��ʯ�ֽ�ĸ���Ҫ��������̼��ƿ��Ժ����ᷢ����Ӧ��
�ʴ�Ϊ������̼���Ҫ�ڸ����²�����ȫ�ֽ⣮
��3���⣺�������������Ʒ�Ӧ��HCl����Ϊx
HCl+NaOH�TNaCl+H2O
36.5 40
x 3.2g
��
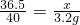
��֮�ã�x=2.92g��
��ô����̼��Ʒ�Ӧ����������Ϊ��7.3%��100g-2.92g=4.38g��
����ʯ��ʯ��̼��Ƶ�����Ϊy��
CaCO3+2HCl=CaCl2+CO2��+H2O
100 73
y 4.38g
��
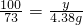
��֮��y=6g��
̼��Ƶ���������Ϊ��
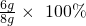 =75%��
=75%���𣺷�������Ʒ��̼��Ƶ���������Ϊ75%��
��������1��������������=��Һ���������������������㼴�ɣ�
��2���������������Կ�������һ�������Ģ����þƾ��Ƽ��ȣ��¶ȴﲻ��ʯ��ʯ�ֽ�ĸ���Ҫ��������̼��ƿ��Ժ����ᷢ����Ӧ��
��3���ٸ�������������Һ�����ᷴӦ�Ļ�ѧ����ʽ��������������Ʒ�Ӧ��HCl������Ȼ�����������HCl������-�������Ʒ�Ӧ��HCl����=��̼��Ʒ�Ӧ��HCl��������
�ڸ���̼��ƺ����ᷴӦ�Ļ�ѧ����ʽ�����̼��Ƶ��������ٸ�������������ʽ���㼴�ɣ�
������������Ҫ����ѧ�����û�ѧ����ʽ������������ʽ���м����������

��ϰ��ϵ�д�
�����Ŀ
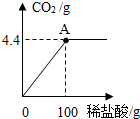 ��2011?���ݣ��������зḻ��ʯ��ʯ��Դ����ѧ��ȤС�����ⶨijʯ��ʯ��Ʒ��CaCO3������������ȡʯ��ʯ��Ʒ15g����������ϡ���ᣨ���ʲ�����ˮ��Ҳ�������ᷴӦ��������ϡ�����������ų�������̼��������ϵ��ͼ��ʾ��������㣺
��2011?���ݣ��������зḻ��ʯ��ʯ��Դ����ѧ��ȤС�����ⶨijʯ��ʯ��Ʒ��CaCO3������������ȡʯ��ʯ��Ʒ15g����������ϡ���ᣨ���ʲ�����ˮ��Ҳ�������ᷴӦ��������ϡ�����������ų�������̼��������ϵ��ͼ��ʾ��������㣺