��Ŀ����
ij��ѧ��ȤС����ʵ������������ʵ��װ�ü�ҩƷ���������ʵ�������Ʊ���������Ķ�����̼�������һ����̼����ⶨij������������Ʒ�Ĵ��ȣ�
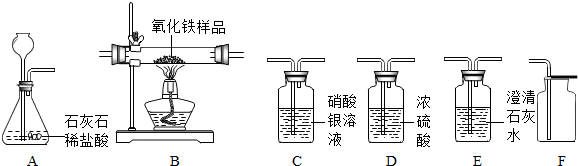
�Ը�����ĿҪ�ش��������⣺������������з����ķ�Ӧ��ǡ����ȫ���У�ʵ���п��ܻӷ�������HCI���壩��
��1����Ҫ�Ʊ����ռ���������Ķ�����̼������������˳��Ϊ
��2��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ
��3�����û�������������̼��һ����̼���壬�ⶨij������������Ʒ�Ĵ��ȣ����ʲ���Ӧ��������֤��Ӧ����������������ʣ�ijͬѧ��ѡ����������˳��Ϊ����������B��E��
������Ӧǰװ��B����Ʒ����Ϊ10g����Ӧ��װ��B�й�������Ϊ7.9g������Ʒ������������������Ϊ
�����÷�Ӧ��װ��E�����ӵ�������������Ʒ�������������������������
�۴ӻ��������ĽǶȿ��ǣ���Ը���ʵ��װ�ò�������ľ���Ľ����

�Ը�����ĿҪ�ش��������⣺������������з����ķ�Ӧ��ǡ����ȫ���У�ʵ���п��ܻӷ�������HCI���壩��
��1����Ҫ�Ʊ����ռ���������Ķ�����̼������������˳��Ϊ
ACDF
ACDF
����2��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ
CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
��3�����û�������������̼��һ����̼���壬�ⶨij������������Ʒ�Ĵ��ȣ����ʲ���Ӧ��������֤��Ӧ����������������ʣ�ijͬѧ��ѡ����������˳��Ϊ����������B��E��
������Ӧǰװ��B����Ʒ����Ϊ10g����Ӧ��װ��B�й�������Ϊ7.9g������Ʒ������������������Ϊ
70%
70%
�������÷�Ӧ��װ��E�����ӵ�������������Ʒ�������������������������
ƫ��
ƫ��
��ѡ�ƫ����ƫС����������Ӱ�족֮һ������˵������һ����̼�л��еĶ�����̼û�г�ȥ����ʹװ��E���յĶ�����̼����ƫ�������ɴ˼������������������ƫ������������Ҳƫ��
һ����̼�л��еĶ�����̼û�г�ȥ����ʹװ��E���յĶ�����̼����ƫ�������ɴ˼������������������ƫ������������Ҳƫ��
���۴ӻ��������ĽǶȿ��ǣ���Ը���ʵ��װ�ò�������ľ���Ľ����
��Eװ�õĶ̹ܴ���һֻ��ȼ�ľƾ���
��Eװ�õĶ̹ܴ���һֻ��ȼ�ľƾ���
����������1��ʵ�������Ʊ����ռ�����Ķ�����̼���壬���ȸ��������ҩƷ��ѡ��Ӧװ�ã�Ȼ���ɶ�����̼��������ѡȡ������������ݶ�����̼���������ʣ��ȿ�������ѡ���ռ�װ�ü�������
��2��װ��A��Ϊ̼�����ϡ���ᷴӦ�������д������Ӧ�Ļ�ѧ����ʽ��
��3���ٸ�����Ŀ�е����ݺͻ�ѧ����ʽ���㣬�ٸ����������Ĵ���=
��100%�����
�����÷�Ӧ��װ��E�����ӵ����������㣬����һ����̼�л��еĶ�����̼û�г�ȥ����ʹװ��E���յĶ�����̼����ƫ��
�۸���һ����̼�ж������Դӻ��������ĽǶȿ��ǣ��������Ľ������
��2��װ��A��Ϊ̼�����ϡ���ᷴӦ�������д������Ӧ�Ļ�ѧ����ʽ��
��3���ٸ�����Ŀ�е����ݺͻ�ѧ����ʽ���㣬�ٸ����������Ĵ���=
| ���������� |
| ��Ʒ���� |
�����÷�Ӧ��װ��E�����ӵ����������㣬����һ����̼�л��еĶ�����̼û�г�ȥ����ʹװ��E���յĶ�����̼����ƫ��
�۸���һ����̼�ж������Դӻ��������ĽǶȿ��ǣ��������Ľ������
����⣺��1��ʵ�������Ʊ����ռ�����Ķ�����̼���壬Ҫѡ�õ�ҩƷ��ϡ�����ʯ��ʯ�����������̼��ҩƷ��Ũ���ᣬ��Ϊ������̼�ȿ����أ�����ѡ�������ſ��������ռ����壻�����Ҫ�õ���װ���Ƿ���װ��A�������Ȼ���������C�����������̼����װ��D���ռ�װ��F��������˳����Ӧ������ȡ���塢�����ʡ��������塢�ռ����壻
��2��װ��A��Ϊ̼�����ϡ���ᷴӦ�����仯ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
��3������������������ΪX��
Fe2O3+3CO
2Fe+3CO2�� ���������仯
160 112 48
X 10g-7.1g=2.1g
=
�����X=7g��
������������������
��100%=70%��
�����÷�Ӧ��װ��E�����ӵ�������������Ʒ������������������������� ƫ������ һ����̼�л��еĶ�����̼û�г�ȥ����ʹװ��E���յĶ�����̼����ƫ�������ɴ˼������������������ƫ������������Ҳƫ��
�۴ӻ��������ĽǶȿ��ǣ���Ը���ʵ��װ�ò�������ľ���Ľ����Ϊ ��Eװ�õĶ̹ܴ���һֻ��ȼ�ľƾ��ƣ�
�ʴ�Ϊ����1��ACDF�� ��2��CaCO3+2HCl=CaCl2+H2O+CO2����
��3����70% ��ƫ�� һ����̼�л��еĶ�����̼û�г�ȥ����ʹװ��E���յĶ�����̼����ƫ�������ɴ˼������������������ƫ������������Ҳƫ�������װ��E���տ����еĶ�����̼�ش𣩣�����Eװ�õĶ̹ܴ���һֻ��ȼ�ľƾ��ƻ�ϵһ�������ռ�β��
��2��װ��A��Ϊ̼�����ϡ���ᷴӦ�����仯ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
��3������������������ΪX��
Fe2O3+3CO
| ||
160 112 48
X 10g-7.1g=2.1g
| 160 |
| 48 |
| x |
| 2.1g |
������������������
| 7g |
| 10g |
�����÷�Ӧ��װ��E�����ӵ�������������Ʒ������������������������� ƫ������ һ����̼�л��еĶ�����̼û�г�ȥ����ʹװ��E���յĶ�����̼����ƫ�������ɴ˼������������������ƫ������������Ҳƫ��
�۴ӻ��������ĽǶȿ��ǣ���Ը���ʵ��װ�ò�������ľ���Ľ����Ϊ ��Eװ�õĶ̹ܴ���һֻ��ȼ�ľƾ��ƣ�
�ʴ�Ϊ����1��ACDF�� ��2��CaCO3+2HCl=CaCl2+H2O+CO2����
��3����70% ��ƫ�� һ����̼�л��еĶ�����̼û�г�ȥ����ʹװ��E���յĶ�����̼����ƫ�������ɴ˼������������������ƫ������������Ҳƫ�������װ��E���տ����еĶ�����̼�ش𣩣�����Eװ�õĶ̹ܴ���һֻ��ȼ�ľƾ��ƻ�ϵһ�������ռ�β��
�����������ۺϿ�����ʵ������ȡ������̼��װ�õ�ѡȡ������˳��һ����̼��ԭ�������Ļ�ѧ��Ӧ�Լ���ѧ����ʽ����д��

��ϰ��ϵ�д�
�����Ŀ
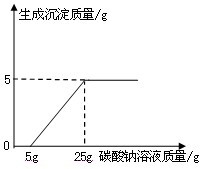 ij��ѧ��ȤС������ʯ��ʯ�����ʲ����ᷴӦ��Ҳ������ˮ����ϡ���ᷴӦ��ȡ������̼����������Ӧ��ķ�Һ������Һ��ʱ������ʵ��������һƿδ֪����������Na2CO3��Һ�����Ǿ������ø÷�Һ�ⶨ��ƿNa2CO3��Һ���������������������Ƚ���Һ���ˣ�Ȼ�����Һ�������μ�Na2CO3��Һ������Na2CO3��Һ�����������ɳ��������Ĺ�ϵ����ͼ��ʾ��
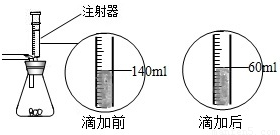
ij��ѧ��ȤС������ʯ��ʯ�����ʲ����ᷴӦ��Ҳ������ˮ����ϡ���ᷴӦ��ȡ������̼����������Ӧ��ķ�Һ������Һ��ʱ������ʵ��������һƿδ֪����������Na2CO3��Һ�����Ǿ������ø÷�Һ�ⶨ��ƿNa2CO3��Һ���������������������Ƚ���Һ���ˣ�Ȼ�����Һ�������μ�Na2CO3��Һ������Na2CO3��Һ�����������ɳ��������Ĺ�ϵ����ͼ��ʾ��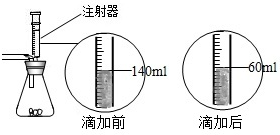 ij��ѧ��ȤС����ʵ�������ȡ������̼����ѡ������ͼ�ķ���װ�ã�����ȡ��Ϻ���ͬѧ�����װ�û��ܲ��ϡ���������������С��ͬѧ��ȡ12.5g��CaCO380%��ʯ��ʯ��ĩ��Ʒ����װ���У���ע�����μ�ϡ������ǡ����ȫ��Ӧ���μ�ǰ�����������ͼ��ʾ����������ϡ�����Ӧ��ϡ������ܶ�Ϊ1.25g/mL��
ij��ѧ��ȤС����ʵ�������ȡ������̼����ѡ������ͼ�ķ���װ�ã�����ȡ��Ϻ���ͬѧ�����װ�û��ܲ��ϡ���������������С��ͬѧ��ȡ12.5g��CaCO380%��ʯ��ʯ��ĩ��Ʒ����װ���У���ע�����μ�ϡ������ǡ����ȫ��Ӧ���μ�ǰ�����������ͼ��ʾ����������ϡ�����Ӧ��ϡ������ܶ�Ϊ1.25g/mL�� ij��ѧ��ȤС����ʵ�������ȡ������̼����ѡ������ͼ�ķ���װ�ã�����ȡ��Ϻ���ͬѧ�����װ�û��ܲ��ϡ���������������С��ͬѧ��ȡ12.5g��CaCO380%��ʯ��ʯ��ĩ��Ʒ����װ���У���ע�����μ�ϡ������ǡ����ȫ��Ӧ���μ�ǰ�����������ͼ��ʾ����������ϡ�����Ӧ��ϡ������ܶ�Ϊ1.25g/mL��
ij��ѧ��ȤС����ʵ�������ȡ������̼����ѡ������ͼ�ķ���װ�ã�����ȡ��Ϻ���ͬѧ�����װ�û��ܲ��ϡ���������������С��ͬѧ��ȡ12.5g��CaCO380%��ʯ��ʯ��ĩ��Ʒ����װ���У���ע�����μ�ϡ������ǡ����ȫ��Ӧ���μ�ǰ�����������ͼ��ʾ����������ϡ�����Ӧ��ϡ������ܶ�Ϊ1.25g/mL��