��Ŀ����
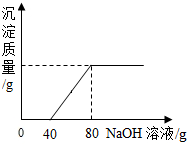
 ��һ�ձ���ʢ��Na2CO3��NaOH�Ļ����10g�������м���100gˮ������ȫ���ܽ⣮��������Һ�еμӳ���ʯ��ˮ���������������������ʯ��ˮ��������ϵ������ͼ��ʾ�����������ش��������⣺
��һ�ձ���ʢ��Na2CO3��NaOH�Ļ����10g�������м���100gˮ������ȫ���ܽ⣮��������Һ�еμӳ���ʯ��ˮ���������������������ʯ��ˮ��������ϵ������ͼ��ʾ�����������ش��������⣺��1���ڵ���ʯ��ˮʱ��������������ɫ��
��ɫ
��ɫ
����2��������ʯ��ˮ��ͼ��A��ʱ���ձ�����Һ�ﺬ�е������ǣ�д��ѧʽ��
Na2CO3��NaOH
Na2CO3��NaOH
����3�����㵱����ʯ��ˮ��ͼ��B��ʱ����Һ�����ʵ�����Ϊ���٣�����������ȷ��0.1g��
��������1��Na2CO3��NaOH�Ļ����Һ�еμӳ���ʯ��ˮ��̼�������������Ʒ�����Ӧ����̼��Ƴ������������ƣ�
��2��������ʯ��ˮ��ͼ��A��ʱ��̼����û�к�����������ȫ��Ӧ��̼������ʣ�࣬�ݴ˽��з������
��3��������ʯ��ˮ��ͼ��B��ʱ��̼���ƺ���������ǡ����ȫ��Ӧ����Һ�е�����Ϊ�������ƣ��������ɵij����ɷ�Ӧ�Ļ�ѧ����ʽ���з������㼴�ɣ�
��2��������ʯ��ˮ��ͼ��A��ʱ��̼����û�к�����������ȫ��Ӧ��̼������ʣ�࣬�ݴ˽��з������
��3��������ʯ��ˮ��ͼ��B��ʱ��̼���ƺ���������ǡ����ȫ��Ӧ����Һ�е�����Ϊ�������ƣ��������ɵij����ɷ�Ӧ�Ļ�ѧ����ʽ���з������㼴�ɣ�
����⣺��1��Na2CO3��NaOH�Ļ����Һ�еμӳ���ʯ��ˮ��̼�������������Ʒ�Ӧ����̼��Ƴ������������ƣ�̼��Ƶ���ɫΪ��ɫ��
��2��������ʯ��ˮ��ͼ��A��ʱ��̼����û�к�����������ȫ��Ӧ��̼������ʣ�࣬���ձ�����Һ���������ƺ�̼���ƵĻ����Һ��������Na2CO3��NaOH��
��3����������Na2CO3������Ϊx����Ӧ���ɵ�NaOH������Ϊy
Na2CO3+Ca��OH��2�TCaCO3��+2NaOH
106 100 80
x 5g y
=
��ã�x=5.3g
=
��ã�y=4g
��Һ�����ʵ�����Ϊ 10g-5.3g+4g=8.7g
�ʴ�Ϊ����1����ɫ����2��Na2CO3��NaOH����3����Һ�����ʵ�����Ϊ8.7g��
��2��������ʯ��ˮ��ͼ��A��ʱ��̼����û�к�����������ȫ��Ӧ��̼������ʣ�࣬���ձ�����Һ���������ƺ�̼���ƵĻ����Һ��������Na2CO3��NaOH��
��3����������Na2CO3������Ϊx����Ӧ���ɵ�NaOH������Ϊy
Na2CO3+Ca��OH��2�TCaCO3��+2NaOH
106 100 80
x 5g y
| 106 |
| 100 |
| X |
| 5g |
| 100 |
| 80 |
| 5g |
| y |
��Һ�����ʵ�����Ϊ 10g-5.3g+4g=8.7g
�ʴ�Ϊ����1����ɫ����2��Na2CO3��NaOH����3����Һ�����ʵ�����Ϊ8.7g��
�����������ѶȲ�������ͼ�η�����Ӧ�Ľ��̡�ץס������һ���ơ�����㡢�յ㡢�յ��ͼ��ı仯���ƣ�������ͼ�����������ݵĺ��塢��ȷ�������й����ݽ�������ǽ�������Ŀ�Ĺؼ����ڣ�

��ϰ��ϵ�д�
�����Ŀ
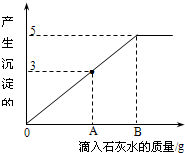
 ��֪Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��20.4g Na2CO3��NaCl��ɵĹ�������������μ�������������Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ��������ͼ��ʾ�����������ش����⣺
��֪Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��20.4g Na2CO3��NaCl��ɵĹ�������������μ�������������Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ��������ͼ��ʾ�����������ش����⣺ ��һ�ձ���ʢ��42.2g CaCO3��CaCl2�ķ�ĩ״���������м���188.8gˮ��ʹ������еĿ�������ȫ�ܽ⣮Ȼ������������μ������ʵ���������Ϊ10%��ϡ���ᣬ�ձ������ܹ������ʵ�������������ϡ�����������ϵ������ͼX��ʾ��
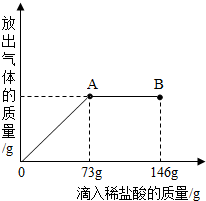
��һ�ձ���ʢ��42.2g CaCO3��CaCl2�ķ�ĩ״���������м���188.8gˮ��ʹ������еĿ�������ȫ�ܽ⣮Ȼ������������μ������ʵ���������Ϊ10%��ϡ���ᣬ�ձ������ܹ������ʵ�������������ϡ�����������ϵ������ͼX��ʾ�� ��2010?���ϣ���һ�ձ���ʢ��һ������������ͭ�������м���������ϡ���ᣬ��ǡ����ȫ��Ӧ����һ���¶��µõ�40gCuSO4�IJ�������Һ����������Һ����ε���������������Ϊ10%��NaOH��Һ����Һ�������������NaOH��Һ��������������ϵ������ͼ��ʾ�����������ش��������⣺
��2010?���ϣ���һ�ձ���ʢ��һ������������ͭ�������м���������ϡ���ᣬ��ǡ����ȫ��Ӧ����һ���¶��µõ�40gCuSO4�IJ�������Һ����������Һ����ε���������������Ϊ10%��NaOH��Һ����Һ�������������NaOH��Һ��������������ϵ������ͼ��ʾ�����������ش��������⣺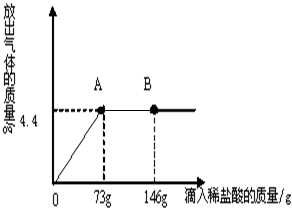 С���ڻ�ѧʵ���ҷ��֣�ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ��С�ս���С����С�죬��ͬ̽�����ְ�ɫ��ĩ�ijɷ֣�
С���ڻ�ѧʵ���ҷ��֣�ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ��С�ս���С����С�죬��ͬ̽�����ְ�ɫ��ĩ�ijɷ֣�