��Ŀ����
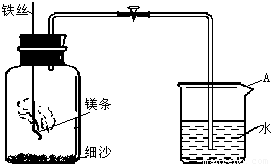
��2011?������һģ��������̽��ˮ����ɵ�ʵ�飮��ͼ�ǵ��ˮʵ���ʾ��ͼ��
��1��A�˽ӵ��______�����������������
��2����ʵ���֤��ˮ��Ԫ����ɣ�ˮ���ɣ�д���ƣ�______��ɵģ�
��3��ˮ��Ԫ�ش�����ʽ��______�������̬������̬������
��4�������ˮ����ˮ3.6g����A�Թ�����������ķ��Ӹ���ԼΪ______��������ʽ���㣩
��5��Ϊ�˽�һ���ⶨˮ�е�Ԫ����ɵ������ȣ�ij �Ƽ�С���ͬѧ���������ʵ�飨װ������ͼ��������������ԭ����ͭ����ͭ��ˮ��ͨ��������Ӧǰ��װ��A��B�������������� m��H����m��O����1��8��������ֵƫ�ߣ���ԭ�������______�������ţ�
A��ͨ�������δ�������� B��װ��A�ڹܿ���ˮ����
C������ͭû����ȫ��ԭ D��װ��Bͬʱ�����˿����е�ˮ������CO2��
���𰸡���������1������2�����ݵ��ˮ��ʵ����������������ش𣬵��ˮ������ɼ���Ϊ��������һ�ȶ��������һ�ȶ�������ȣ�
��3��Ԫ���ڻ��������Ի���̬���ڣ��ڵ�����������̬���ڣ�
��4�����ݵ��ˮ�Ļ�ѧ����ʽ���м��㣬
��5����ʵ�������ʧ�����ֿ��ǣ�
����⣺
��1����ͼ��֪��A�Թ����������������������Ϊ����������������A�����ӵ�Դ�ĸ�����
��2������������������������֤��ˮ��Ԫ����ɣ�ˮ������Ԫ�غ���Ԫ����ɵģ�
��3����Ϊˮ���ڻ��������ˮ�е�Ԫ���Ի���̬��ʽ���ڣ�
��4�������3.6gˮ�������ɵ�������Ħ������Ϊx
2H2O 2H2��+O2��
2H2��+O2��
36 2
3.6g x

���x=0.2g ���ʵ����ǣ�0.2mol
0.2mol����ķ��Ӹ�����1.204×1023
��5��������m��H����m��O��������ֵƫ�ߣ���ͨ��U�ܵ����أ�ˮ����������Ӳ���Թܵ������ļ�С����������������ԭ�������ͨ�������δ��������ʱ���ܵ�����ʯ�����ؽ϶࣬����ʱ���������� m��H����m��O����1��8��װ��װ��B�������˿����еĶ�����̼��ˮ���������¼���ʱ������ʵ��ֵ�ϴ�����ˮ��û����ȫ�����գ�������ͭ�Ƿ���ȫ��Ӧ������û��Ӱ�죬��ѡABD��
������1����
��2����Ԫ�غ���Ԫ��
��3������̬
��4��1.204×1023
��5��ABD
�����������ȫ��Ŀ����˵��ˮ��ʵ������ʵ���ʵ�ʣ�����ļ��飬�йػ�ѧ����ʽ�ļ���ȣ���һ���ۺ��ԱȽ�ǿ��ϰ�⣮
��3��Ԫ���ڻ��������Ի���̬���ڣ��ڵ�����������̬���ڣ�
��4�����ݵ��ˮ�Ļ�ѧ����ʽ���м��㣬
��5����ʵ�������ʧ�����ֿ��ǣ�
����⣺
��1����ͼ��֪��A�Թ����������������������Ϊ����������������A�����ӵ�Դ�ĸ�����
��2������������������������֤��ˮ��Ԫ����ɣ�ˮ������Ԫ�غ���Ԫ����ɵģ�
��3����Ϊˮ���ڻ��������ˮ�е�Ԫ���Ի���̬��ʽ���ڣ�
��4�������3.6gˮ�������ɵ�������Ħ������Ϊx
2H2O
 2H2��+O2��
2H2��+O2��36 2
3.6g x

���x=0.2g ���ʵ����ǣ�0.2mol
0.2mol����ķ��Ӹ�����1.204×1023
��5��������m��H����m��O��������ֵƫ�ߣ���ͨ��U�ܵ����أ�ˮ����������Ӳ���Թܵ������ļ�С����������������ԭ�������ͨ�������δ��������ʱ���ܵ�����ʯ�����ؽ϶࣬����ʱ���������� m��H����m��O����1��8��װ��װ��B�������˿����еĶ�����̼��ˮ���������¼���ʱ������ʵ��ֵ�ϴ�����ˮ��û����ȫ�����գ�������ͭ�Ƿ���ȫ��Ӧ������û��Ӱ�죬��ѡABD��
������1����
��2����Ԫ�غ���Ԫ��
��3������̬
��4��1.204×1023
��5��ABD
�����������ȫ��Ŀ����˵��ˮ��ʵ������ʵ���ʵ�ʣ�����ļ��飬�йػ�ѧ����ʽ�ļ���ȣ���һ���ۺ��ԱȽ�ǿ��ϰ�⣮

��ϰ��ϵ�д�
�����Ŀ

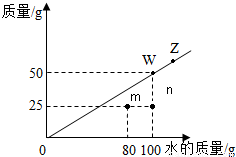 ��2011?������һģ��t��ʱ������ij�����ڲ�ͬ������ˮ�дﵽ����״̬ʱ���ܽ���������Ƴ���ͼ�е�б�ߣ�����˵����ȷ���ǣ� ��
��2011?������һģ��t��ʱ������ij�����ڲ�ͬ������ˮ�дﵽ����״̬ʱ���ܽ���������Ƴ���ͼ�е�б�ߣ�����˵����ȷ���ǣ� ��