��Ŀ����
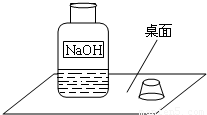
һ�죬С�����ʦһ���߽�ʵ���ң����ÿ��ʵ�����ϵ�ҩƷ�������Ƿ��뱸�������߽�ij���ʱ������һ������г�ġ�����������ͼ��ʾ����1����������龰�������뵽��������������Һ���ܱ����ˣ��ñ��ʷ�Ӧ�Ļ�ѧ����ʽΪ��______��
��2��Χ�ƴ�ƿNaOH��Һ�Ƿ���ʵ����⣬С������ʵ���ҵ������Լ����Ȼ�����Һ��ϡ���ᡢ��̪��Һ��չ��������̽�����
��ȡ������Һ���Թ��У��μ�ij���Լ��������ݲ������ɴ�֤��NaOH��Һ�Ѿ����ʣ�����ΪС�����ӵ��Լ���______��֤��NaOH��Һ�Ѿ����ʵķ�Ӧ�Ļ�ѧ����ʽΪ______��
����֤�����ʵ���Һ���д�NaOH��������д�����йؿո���С�����̽��������
| ̽��Ŀ�� | ̽������ | Ԥ������ |
| 1��������Һ�е�CO32- | A��ȡ������Һ���Թ��У� �μ�������______�� | B����______���� |
| 2��֤����Һ���д�NaOH | ��ʵ��A������Һ�е� ��______�� | C��______�� |
���𰸡���������1��NaOH��Һ���տ����еĶ�����̼����Na2CO3��
��2����H2SO4+Na2CO3=Na2SO4+CO2��+H2O��
��Na2CO3+CaCl2=CaCO3��+2NaCl����̪������ɫ�������Һ���д�NaOH����ô������NaOH��Һ�м�������CaCl2��Һ���ټӷ�̪��Һ��Ȳ�����ɫ�����ֱ��ɫ�������Һ�в�����NaOH����������CaCl2��Һ��ͳ�����CO32-��û������������Ҳû����̼����ֻ������ɫ�����ټӷ�̪��Һ����ɫ��
����⣺��1��NaOH��Һ�����տ����еĶ�����̼����Na2CO3
�ʸñ��ʷ�Ӧ�ķ���ʽΪ��2NaOH+CO2�TNa2CO3+H2O
��2����ϡ�������̼���Ʒ�Ӧ���ɶ�����̼���壬H2SO4+Na2CO3=Na2SO4+CO2��+H2O��
��Na2CO3+CaCl2=CaCO3��+2NaCl����̪������ɫ�������Һ���д�NaOH����ô������NaOH��Һ�м�������CaCl2��Һ���ټӷ�̪��Һ��Ȳ�����ɫ�����ֱ��ɫ�������Һ�в�����NaOH����������CaCl2��Һ��ͳ�����CO32-��û������������Ҳû����̼�����ټӷ�̪��Һֻ������ɫ����������ɫ��
�ʴ�Ϊ����1��2NaOH+CO2�TNa2CO3+H2O��
��2����ϡ���ᣬH2SO4+Na2CO3=Na2SO4+CO2��+H2O��
��
�����������ۺϿ������������Ƶ������Լ�̼����ļ��鷽�������ӷ�����ע����ռ����Լ����������ӹ����в��ܼ����µ��������ӣ���ȷѡ���Լ���
��2����H2SO4+Na2CO3=Na2SO4+CO2��+H2O��
��Na2CO3+CaCl2=CaCO3��+2NaCl����̪������ɫ�������Һ���д�NaOH����ô������NaOH��Һ�м�������CaCl2��Һ���ټӷ�̪��Һ��Ȳ�����ɫ�����ֱ��ɫ�������Һ�в�����NaOH����������CaCl2��Һ��ͳ�����CO32-��û������������Ҳû����̼����ֻ������ɫ�����ټӷ�̪��Һ����ɫ��
����⣺��1��NaOH��Һ�����տ����еĶ�����̼����Na2CO3
�ʸñ��ʷ�Ӧ�ķ���ʽΪ��2NaOH+CO2�TNa2CO3+H2O
��2����ϡ�������̼���Ʒ�Ӧ���ɶ�����̼���壬H2SO4+Na2CO3=Na2SO4+CO2��+H2O��
��Na2CO3+CaCl2=CaCO3��+2NaCl����̪������ɫ�������Һ���д�NaOH����ô������NaOH��Һ�м�������CaCl2��Һ���ټӷ�̪��Һ��Ȳ�����ɫ�����ֱ��ɫ�������Һ�в�����NaOH����������CaCl2��Һ��ͳ�����CO32-��û������������Ҳû����̼�����ټӷ�̪��Һֻ������ɫ����������ɫ��
�ʴ�Ϊ����1��2NaOH+CO2�TNa2CO3+H2O��
��2����ϡ���ᣬH2SO4+Na2CO3=Na2SO4+CO2��+H2O��
��
| ������Һ�е�CO32- | �Ȼ�����Һ | �а�ɫ�������� |
| ��̪��Һ | ��Һ��� |
�����������ۺϿ������������Ƶ������Լ�̼����ļ��鷽�������ӷ�����ע����ռ����Լ����������ӹ����в��ܼ����µ��������ӣ���ȷѡ���Լ���

��ϰ��ϵ�д�
�����Ŀ
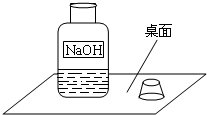 һ�죬С�����ʦһ���߽�ʵ���ң����ÿ��ʵ�����ϵ�ҩƷ�������Ƿ��뱸�������߽�ij���ʱ������һ������г�ġ�����������ͼ��ʾ��
һ�죬С�����ʦһ���߽�ʵ���ң����ÿ��ʵ�����ϵ�ҩƷ�������Ƿ��뱸�������߽�ij���ʱ������һ������г�ġ�����������ͼ��ʾ��
��1����������龰�������뵽��������������Һ���ܱ����ˣ��ñ��ʷ�Ӧ�Ļ�ѧ����ʽΪ��______��
��2��Χ�ƴ�ƿNaOH��Һ�Ƿ���ʵ����⣬С������ʵ���ҵ������Լ����Ȼ�����Һ��ϡ���ᡢ��̪��Һ��չ��������̽�����
��ȡ������Һ���Թ��У��μ�ij���Լ��������ݲ������ɴ�֤��NaOH��Һ�Ѿ����ʣ�����ΪС�����ӵ��Լ���______��֤��NaOH��Һ�Ѿ����ʵķ�Ӧ�Ļ�ѧ����ʽΪ______��
����֤�����ʵ���Һ���д�NaOH��������д�����йؿո���С�����̽��������
| ̽��Ŀ�� | ̽������ | Ԥ������ |
| 1��������Һ�е�CO32- | A��ȡ������Һ���Թ��У� �μ�������______�� | B����______���� |
| 2��֤����Һ���д�NaOH | ��ʵ��A������Һ�е� ��______�� | C��______�� |
 26��һ�죬ʵ������С���߽�ʵ���ң�����ʦһ����ÿ��ʵ�����ϵ�ҩƷ�������Ƿ��뱸���ߵ�ij���ʱ������һ������г�ġ�������������ͼ����
26��һ�죬ʵ������С���߽�ʵ���ң�����ʦһ����ÿ��ʵ�����ϵ�ҩƷ�������Ƿ��뱸���ߵ�ij���ʱ������һ������г�ġ�������������ͼ����
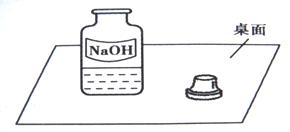 15��һ�죬С�����ʦһ���߽�ʵ���ң����ÿ��ʵ�����ϵ�ҩƷ�������Ƿ��뱸�������߽�ij���ʱ������һ������г�ġ�����������ͼ��ʾ��
15��һ�죬С�����ʦһ���߽�ʵ���ң����ÿ��ʵ�����ϵ�ҩƷ�������Ƿ��뱸�������߽�ij���ʱ������һ������г�ġ�����������ͼ��ʾ��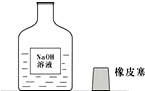 һ�죬ʵ������С���߽�ʵ���ң�����ʦһ����ÿ��ʵ�����ϵ�ҩƷ�������Ƿ��뱸���ߵ�ij���ʱ������һ������г�ġ�����������ͼ��
һ�죬ʵ������С���߽�ʵ���ң�����ʦһ����ÿ��ʵ�����ϵ�ҩƷ�������Ƿ��뱸���ߵ�ij���ʱ������һ������г�ġ�����������ͼ��