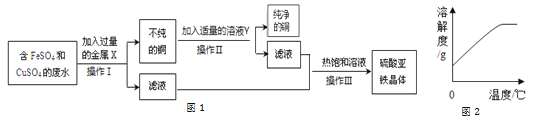
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΫαΚœΆΦ1 Β―ιΉΑ÷ΟΘ§ΜΊ¥πΘΚ

![]() Β―ι “»τ”ΟΖ÷ΫβΑΒΉœ…ΪΙΧΧε÷Τ»Γ
Β―ι “»τ”ΟΖ÷ΫβΑΒΉœ…ΪΙΧΧε÷Τ»Γ![]() Θ§ Β―ιΉΑ÷Ο «______Θ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______ΓΘ
Θ§ Β―ιΉΑ÷Ο «______Θ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______ΓΘ
![]() ΧΫΨΩ
ΧΫΨΩ![]() –‘÷ ΒΡΉΑ÷ΟΔώΓΔΔρ
–‘÷ ΒΡΉΑ÷ΟΔώΓΔΔρ![]() »γΆΦ
»γΆΦ![]()
ΔΌΉΑ÷ΟΔώΘ§ΫΪY–ΈΒΦΙήΤΫΖ≈”ΎΉάΟφ…œΘ§Ά®»κ![]() ΚσΘ§Ιέ≤λΒΫaΙή÷– ‘÷Ϋ±δΚλ…ΪΘ§bΙή÷– ‘÷ΫΈόΟςœ‘±δΜ·Θ§¥Υœ÷œσΥΒΟς
ΚσΘ§Ιέ≤λΒΫaΙή÷– ‘÷Ϋ±δΚλ…ΪΘ§bΙή÷– ‘÷ΫΈόΟςœ‘±δΜ·Θ§¥Υœ÷œσΥΒΟς![]() Ρή”κ______Ζ¥”ΠΘ§…ζ≥…ΒΡ______ ΙΉœ…Ϊ ·»ο±δΚλ…ΪΓΘ
Ρή”κ______Ζ¥”ΠΘ§…ζ≥…ΒΡ______ ΙΉœ…Ϊ ·»ο±δΚλ…ΪΓΘ
ΔΎΉΑ÷ΟΔρΘ§ΫΪY–ΈΒΦΙήΙΧΕ®‘ΎΧζΦήΧ®…œΘ§aΙή‘Ύ…œΖΫΘ§bΙή‘Ύœ¬ΖΫΘ§Ά®»κ![]() ΚσΘ§Ιέ≤λΒΫbΙή÷– ‘÷Ϋœ»±δΚλ…Ϊ«“―’…ΪΫœ«≥…νΘ§¥Υœ÷œσΥΒΟς
ΚσΘ§Ιέ≤λΒΫbΙή÷– ‘÷Ϋœ»±δΚλ…Ϊ«“―’…ΪΫœ«≥…νΘ§¥Υœ÷œσΥΒΟς![]() ΨΏ”–ΔΌΥυ―ι÷ΛΒΡ–‘÷ ΆβΘ§ΜΙΥΒΟς
ΨΏ”–ΔΌΥυ―ι÷ΛΒΡ–‘÷ ΆβΘ§ΜΙΥΒΟς![]() ΒΡΝμ“Μ÷÷–‘÷ Θ§Υϋ «______ΓΘ
ΒΡΝμ“Μ÷÷–‘÷ Θ§Υϋ «______ΓΘ
![]() Β―ι “÷Τ»Γ
Β―ι “÷Τ»Γ![]() ΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______Θ§”Π―Γ______
ΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______Θ§”Π―Γ______![]() ΧνΉ÷ΡΗ
ΧνΉ÷ΡΗ![]() ΉΑ÷ΟΘ§―Γ‘ώΗΟΉΑ÷ΟΒΡάμ”… «______ΓΘ
ΉΑ÷ΟΘ§―Γ‘ώΗΟΉΑ÷ΟΒΡάμ”… «______ΓΘ
![]() Φλ―ιΕΰ―θΜ·ΧΦΒΡΖ¥”ΠΜ·―ßΖΫ≥Χ ΫΈΣ______ΓΘ
Φλ―ιΕΰ―θΜ·ΧΦΒΡΖ¥”ΠΜ·―ßΖΫ≥Χ ΫΈΣ______ΓΘ
ΓΨ¥πΑΗΓΩB 2KMnO4![]() K2MnO4+MnO2+O2
K2MnO4+MnO2+O2![]() Υ° ΧΦΥα ΟήΕ»±»Ω’Τχ¥σ
Υ° ΧΦΥα ΟήΕ»±»Ω’Τχ¥σ ![]() A Ζ¥”ΠΈοΈΣΙΧΧεΚΆ“ΚΧε«“≤Μ–η“ΣΦ”»»Θ§Ά§ ±…ζ≥…ΒΡΤχΧεΟήΕ»±»Ω’Τχ¥σ
A Ζ¥”ΠΈοΈΣΙΧΧεΚΆ“ΚΧε«“≤Μ–η“ΣΦ”»»Θ§Ά§ ±…ζ≥…ΒΡΤχΧεΟήΕ»±»Ω’Τχ¥σ ![]()
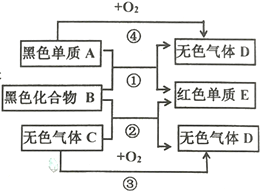
ΓΨΫβΈωΓΩ
![]() »γΙϊ”ΟΑΒΉœ…ΪΒΡΗΏΟΧΥαΦΊ÷Τ―θΤχΨΆ–η“ΣΦ”»»Θ§ΗΏΟΧΥαΦΊ ή»»Ζ÷Ϋβ…ζ≥…ΟΧΥαΦΊΚΆΕΰ―θΜ·ΟΧΚΆ―θΤχΘ§“ΣΉΔ“β≈δΤΫΘΜ
»γΙϊ”ΟΑΒΉœ…ΪΒΡΗΏΟΧΥαΦΊ÷Τ―θΤχΨΆ–η“ΣΦ”»»Θ§ΗΏΟΧΥαΦΊ ή»»Ζ÷Ϋβ…ζ≥…ΟΧΥαΦΊΚΆΕΰ―θΜ·ΟΧΚΆ―θΤχΘ§“ΣΉΔ“β≈δΤΫΘΜ
Ι ¥πΑΗΈΣΘΚBΘΜ2KMnO4![]() K2MnO4+MnO2+O2
K2MnO4+MnO2+O2![]() ΘΜ
ΘΜ
![]() ΆΦ2÷–Θ§ΔΌΉΑ÷ΟΔώΘ§ΫΪY–ΈΒΦΙήΤΫΖ≈”ΎΉάΟφ…œΘ§Ά®»κ
ΆΦ2÷–Θ§ΔΌΉΑ÷ΟΔώΘ§ΫΪY–ΈΒΦΙήΤΫΖ≈”ΎΉάΟφ…œΘ§Ά®»κ![]() ΚσΘ§Ιέ≤λΒΫaΙή÷– ‘÷Ϋ±δΚλ…ΪΘ§bΙή÷– ‘÷ΫΈόΟςœ‘±δΜ·Θ§¥Υœ÷œσΥΒΟς
ΚσΘ§Ιέ≤λΒΫaΙή÷– ‘÷Ϋ±δΚλ…ΪΘ§bΙή÷– ‘÷ΫΈόΟςœ‘±δΜ·Θ§¥Υœ÷œσΥΒΟς![]() Ρή”κΥ°Ζ¥”ΠΘ§…ζ≥…ΧΦΥαΘΜΔΎΉΑ÷ΟΔρΘ§ΫΪY–ΈΒΦΙήΙΧΕ®‘ΎΧζΦήΧ®…œΘ§aΙή‘Ύ…œΖΫΘ§bΙή‘Ύœ¬ΖΫΘ§Ά®»κ
Ρή”κΥ°Ζ¥”ΠΘ§…ζ≥…ΧΦΥαΘΜΔΎΉΑ÷ΟΔρΘ§ΫΪY–ΈΒΦΙήΙΧΕ®‘ΎΧζΦήΧ®…œΘ§aΙή‘Ύ…œΖΫΘ§bΙή‘Ύœ¬ΖΫΘ§Ά®»κ![]() ΚσΘ§Ιέ≤λΒΫbΙή÷– ‘÷Ϋœ»±δΚλ…Ϊ«“―’…ΪΫœ«≥…νΘ§¥Υœ÷œσΥΒΟς
ΚσΘ§Ιέ≤λΒΫbΙή÷– ‘÷Ϋœ»±δΚλ…Ϊ«“―’…ΪΫœ«≥…νΘ§¥Υœ÷œσΥΒΟς![]() ΨΏ”–ΔΎΥυ―ι÷ΛΒΡ–‘÷ ΆβΘ§ΜΙΥΒΟς
ΨΏ”–ΔΎΥυ―ι÷ΛΒΡ–‘÷ ΆβΘ§ΜΙΥΒΟς![]() ΒΡΟήΕ»±»Ω’Τχ¥σΘΜ
ΒΡΟήΕ»±»Ω’Τχ¥σΘΜ
Ι ¥πΑΗΈΣΘΚΔΌΥ°ΓΔΧΦΥαΘΜΔΎΟήΕ»±»Ω’Τχ¥σΘΜ
![]() Β―ι “÷Τ»Γ
Β―ι “÷Τ»Γ![]() Θ§ «‘Ύ≥ΘΈ¬œ¬Θ§”Ο¥σάμ ·Μρ ·Μ“ ·ΚΆœΓ―ΈΥα÷Τ»ΓΒΡΘ§ΧΦΥαΗΤΚΆ―ΈΥαΖ¥”Π…ζ≥…¬»Μ·ΗΤΚΆΥ°ΚΆΕΰ―θΜ·ΧΦΘ§“ρ¥Υ≤Μ–η“ΣΦ”»»ΘΜ―Γ‘ώAΉΑ÷ΟΒΡάμ”… «ΘΚΖ¥”ΠΈοΈΣΙΧΧεΚΆ“ΚΧε«“≤Μ–η“ΣΦ”»»Θ§Ά§ ±…ζ≥…ΒΡΤχΧεΟήΕ»±»Ω’Τχ¥σΘΜ
Θ§ «‘Ύ≥ΘΈ¬œ¬Θ§”Ο¥σάμ ·Μρ ·Μ“ ·ΚΆœΓ―ΈΥα÷Τ»ΓΒΡΘ§ΧΦΥαΗΤΚΆ―ΈΥαΖ¥”Π…ζ≥…¬»Μ·ΗΤΚΆΥ°ΚΆΕΰ―θΜ·ΧΦΘ§“ρ¥Υ≤Μ–η“ΣΦ”»»ΘΜ―Γ‘ώAΉΑ÷ΟΒΡάμ”… «ΘΚΖ¥”ΠΈοΈΣΙΧΧεΚΆ“ΚΧε«“≤Μ–η“ΣΦ”»»Θ§Ά§ ±…ζ≥…ΒΡΤχΧεΟήΕ»±»Ω’Τχ¥σΘΜ
Ι ¥πΑΗΈΣΘΚ![]() ΘΜAΘΜΖ¥”ΠΈοΈΣΙΧΧεΚΆ“ΚΧε«“≤Μ–η“ΣΦ”»»Θ§Ά§ ±…ζ≥…ΒΡΤχΧεΟήΕ»±»Ω’Τχ¥σΘΜ
ΘΜAΘΜΖ¥”ΠΈοΈΣΙΧΧεΚΆ“ΚΧε«“≤Μ–η“ΣΦ”»»Θ§Ά§ ±…ζ≥…ΒΡΤχΧεΟήΕ»±»Ω’Τχ¥σΘΜ
![]() Εΰ―θΜ·ΧΦ“ΜΑψ”Ο≥Έ«εΒΡ ·Μ“Υ°Φλ―ιΘΚΑ―ΤχΧεΆ®»κ≥Έ«εΒΡ ·Μ“Υ°÷–Θ§ ·Μ“Υ°±δΜκΉ«Θ§ΨΆ÷ΛΟς «Εΰ―θΜ·ΧΦΘΜ
Εΰ―θΜ·ΧΦ“ΜΑψ”Ο≥Έ«εΒΡ ·Μ“Υ°Φλ―ιΘΚΑ―ΤχΧεΆ®»κ≥Έ«εΒΡ ·Μ“Υ°÷–Θ§ ·Μ“Υ°±δΜκΉ«Θ§ΨΆ÷ΛΟς «Εΰ―θΜ·ΧΦΘΜ
Ι ¥πΑΗΈΣΘΚ![]() ΓΘ
ΓΘ

 ”Δ”ο–Γ”Δ–έΧλΧλΡ§–¥œΒΝ–¥πΑΗ
”Δ”ο–Γ”Δ–έΧλΧλΡ§–¥œΒΝ–¥πΑΗ νΦΌΉς“ΒΑ≤Μ’…ΌΡξΕυΆ·≥ωΑφ…γœΒΝ–¥πΑΗ
νΦΌΉς“ΒΑ≤Μ’…ΌΡξΕυΆ·≥ωΑφ…γœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ–Γ”ξ‘ΡΕΝΩΈΆβΉ ΝœΒΟ÷Σ:¬»ΥαΦΊΒΡΖ÷ΫβΩ…”ΟΕΰ―θΜ·ΟΧΓΔ―θΜ·Ά≠Β»Έο÷ Ής¥ΏΜ·ΦΝΓΘ”Ύ «Θ§ΥϊΕ‘”Αœλ¬»ΥαΦΊΖ÷ΫβΒΡ“ρΥΊΦΑ¥ΏΜ·ΦΝΒΡ¥ΏΜ·–ßΙϊ≤ζ…ζΝΥΧΫΨΩ–Υ»ΛΓΘ
Θ®Χα≥ωΈ ΧβΘ©―θΜ·Ά≠ «Ζώ±»Εΰ―θΜ·ΟΧ¥ΏΜ·–ßΙϊΗϋΚΟΘΩ”Αœλ¬»ΥαΦΊΖ÷ΫβΥΌ¬ ΒΡ“ρΥΊ”–ΡΡ–© ΡΊΘΩ
Θ®…ηΦΤ Β―ι1–Γ”ξ“‘…ζ≥…Β»ΧεΜΐΒΡ―θΤχΈΣ±ξΉΦΘ§…ηΦΤΝΥœ¬Ν–ΦΗΉι Β―ιΓΘ
–ρΚ≈ | KClO3ΒΡ÷ ΝΩ | ΤδΥϊΈο÷ ΒΡ÷ ΝΩ | Έ¬Ε» | ―θΤχΒΡΧεΜΐ | Ζ¥”ΠΥυ–η ±Φδ |
ΔΌ | 10.0 g | 330Γφ | 100 mL | t1 | |
ΔΎ | 10.0 g | CuO 1.5 g | 330Γφ | 100 mL | t2 |
Δέ | 10.0 g | MnO2 1.5 g | 330Γφ | 100 mL | t3 |
Δή | 10.0 g | MnO2 g | 380Γφ | 100mL | t4 |
Θ®1Θ©»τt1ΘΨt2Θ§ΥΒΟς―θΜ·Ά≠ΡήΦ”Ω묻ΥαΦΊΒΡΖ÷ΫβΥΌ¬ ΓΘ»τ“Σ»ΖΕ®―θΜ·Ά≠ «¥ΥΖ¥”ΠΒΡ¥ΏΜ·ΦΝΘ§ΜΙ–ηΧΫΨΩΖ¥”Π«ΑΚσΘ§―θΜ·Ά≠ΒΡ_________ΚΆ_________≤Μ±δΓΘ
Θ®2Θ©–¥≥ω Β―ιΔήΥυ…φΦΑΒΡΜ·―ßΖΫ≥Χ Ϋ__________________ΓΘ
Θ®3Θ© Β―ιΔή÷–MnO2ΒΡ÷ ΝΩΈΣ_________gΘ§»τt3ΘΨt4Θ§‘ρΜ·―ßΖ¥”ΠΩλ¬ΐ”κΈ¬Ε»ΒΡΙΊœΒ «_________ΓΘ
Θ®4Θ©¬»ΥαΦΊΒΡΖ÷ΫβΥΌ¬ Ω…ΡήΜΙ”κ_________“ρΥΊ”–ΙΊΘ§«κ…ηΦΤ Β―ι÷ΛΟς_______ΓΘ