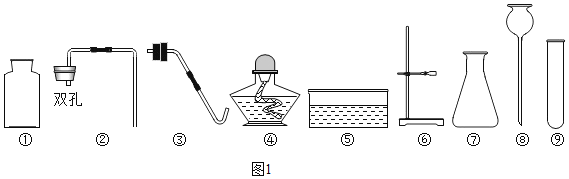
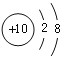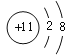��Ŀ����
����Ŀ����ѧ����������̫���ܼ���������CO2�������ͷ�CO2����ʵ��̼ѭ����
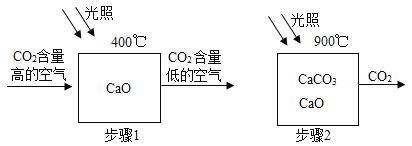
��1������1�Ļ�ѧ����ʽΪ��_______����
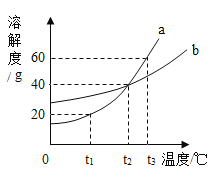
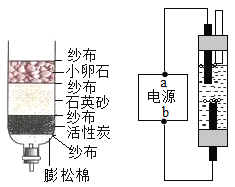
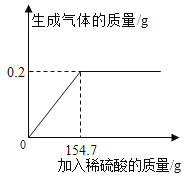
��2��Ϊȷ������2�е�̼����Ƿ���ȫ�ֽ⣬��Ƶ�ʵ�鲽��Ϊ��ȡ�����������Թ��У��μӹ���_______��Һ�����۲쵽�Թ���_______����ֽⲻ��ȫ��
��3������������ŵ���_______������ĸ��ţ���
a ԭ�����ҿ�ѭ������ b �������̫���� c ��ȫ����ȫ���ʹ��
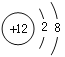
��4��CO2�DZ����̼����Դ��CO2��H2��һ�������¿ɺϳɼ��ᣨHCOOH�����˷�Ӧ��CO2��H2�ķ��Ӹ�����Ϊ_______����Ҫ����1%����ˮ��Һ�Ƿ������ԣ���ѡ�õ���_______������ĸ��ţ�
a ��ɫ��̪��Һ b ��ɫʯ����Һ c pH��ֽ
���𰸡�CaO��CO2 ![]() CaCO3 ϡ���� �����ݲ��� ab 1:1 bc
CaCO3 ϡ���� �����ݲ��� ab 1:1 bc
��������
������Ҫ̽��������̼�Ŀ�ѭ������ʵ������֪ʶ
��1����ͼ��֪��CaO��CO2 ![]() CaCO3
CaCO3
��2��ʹ��ϡ����
��3�������Ե�ʵ���������ݲ���
��4������������ԭ�ϣ����õ��ҿ�ѭ�����ã���Ӧ�����ǹ��գ��������̫���ܣ�ҹ��û�й���ʱ����ʹ��
��5���ɼ���Ļ�ѧʽ��HCOOH����֪��CO2��H2�ķ��Ӹ�����Ϊ1:1
��6����ɫʯ����Һ�������죬pH��ֽ�������Һ��pH<7����ɫ��̪�������������Һ�����ܼ���������Һ

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�