��Ŀ����
����ά���������������������Ԫ�أ���ͼ��ʾΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣�ȡ1Ƭ�ƶ��棬����ʢ��10gϡ������ձ��У����е�̼��Ƹ�ϡ����ǡ����ȫ��Ӧ�������ɷ���ϡ�����Ӧ�����ձ������ʵ�����Ϊ11.34g��������㣺��1��ÿƬ�ƶ����к�̼��Ƶ�������
��2��ʹ�����ֲ��Ƽ���ÿ��ÿ�������Ԫ�ص�������
��3������ϡ���������ʵ�����������

���𰸡���������1������ȷ����⣬�����������̼��Ʒ�Ӧ�Ļ�ѧ����ʽ���ó�������֮��������ȣ��г�����ʽ���������ÿƬ�ƶ����к�̼��Ƶ�������
��2������ȷ����⣬���ȸ���Ԫ�ص�����������ʽ�����̼����и�Ԫ�ص�������������˵�����֪��ʹ�����ֲ��Ƽ���ÿ��ÿ�������2Ƭ����ÿ��ÿ�������Ԫ�ص�����=ÿƬ�ƶ�����CaCO3������×2Ƭ×̼����и�Ԫ�ص������������ݴ˴��⣮
��3���ɣ�1���Ѽ����10g������HCl������������������������=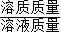 ×100%���ɼ��������������HCl������������
×100%���ɼ��������������HCl������������
����⣺��1����ÿƬ�ƶ�����CaCO3������Ϊx��10g������HCl������Ϊy��
��ȫ��Ӧ������Ķ�����̼������Ϊ��2g+10g-11.34g=0.66g
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
x y 0.66g
��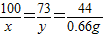
��֮�ã�x=1.5g��y=1.095g��
��2��̼����и�Ԫ�ص���������Ϊ��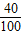 ×100%=40%
×100%=40%
ÿ������ĸƶ����и�Ԫ�ص�����Ϊ��1.5g×2×40%=1.2g��
��3������������HCl����������Ϊ��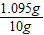 ×100%=10.95%��
×100%=10.95%��
��ÿƬ�ƶ����к�̼��Ƶ�����Ϊ1.5g��ʹ�����ֲ��Ƽ���ÿ��ÿ�������Ԫ�ص�����Ϊ1.2g������ϡ���������ʵ���������Ϊ10.95%��
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м����������ѧ�������������֪�����е�������ϵ����ȷ��д��ѧ����ʽ�����ܽ��
��2������ȷ����⣬���ȸ���Ԫ�ص�����������ʽ�����̼����и�Ԫ�ص�������������˵�����֪��ʹ�����ֲ��Ƽ���ÿ��ÿ�������2Ƭ����ÿ��ÿ�������Ԫ�ص�����=ÿƬ�ƶ�����CaCO3������×2Ƭ×̼����и�Ԫ�ص������������ݴ˴��⣮
��3���ɣ�1���Ѽ����10g������HCl������������������������=
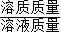 ×100%���ɼ��������������HCl������������
×100%���ɼ��������������HCl����������������⣺��1����ÿƬ�ƶ�����CaCO3������Ϊx��10g������HCl������Ϊy��
��ȫ��Ӧ������Ķ�����̼������Ϊ��2g+10g-11.34g=0.66g
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
x y 0.66g
��
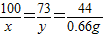
��֮�ã�x=1.5g��y=1.095g��
��2��̼����и�Ԫ�ص���������Ϊ��
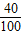 ×100%=40%
×100%=40%ÿ������ĸƶ����и�Ԫ�ص�����Ϊ��1.5g×2×40%=1.2g��
��3������������HCl������������
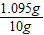 ×100%=10.95%��
×100%=10.95%����ÿƬ�ƶ����к�̼��Ƶ�����Ϊ1.5g��ʹ�����ֲ��Ƽ���ÿ��ÿ�������Ԫ�ص�����Ϊ1.2g������ϡ���������ʵ���������Ϊ10.95%��
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м����������ѧ�������������֪�����е�������ϵ����ȷ��д��ѧ����ʽ�����ܽ��

��ϰ��ϵ�д�
 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
�����Ŀ
 ����ά���������������������Ԫ�أ���ͼ��ʾΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣�ȡ1Ƭ�ƶ��棬����ʢ��10gϡ������ձ��У�����̼��Ƹ�����ǡ����ȫ��Ӧ�������ɷ��������Ӧ�����ձ�������������Ϊ11.34g���Լ��㣺
����ά���������������������Ԫ�أ���ͼ��ʾΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣�ȡ1Ƭ�ƶ��棬����ʢ��10gϡ������ձ��У�����̼��Ƹ�����ǡ����ȫ��Ӧ�������ɷ��������Ӧ�����ձ�������������Ϊ11.34g���Լ��㣺 ����ά���������������������Ԫ�أ���ͼ��ʾΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣��Խ����йصļ��㣺
����ά���������������������Ԫ�أ���ͼ��ʾΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣��Խ����йصļ��㣺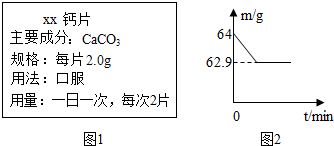 ��2009?����������ά���������������������Ԫ�أ�С�����õ�ij�ָ�Ƭ�IJ���˵����ͼ1��������֪��ÿ����õĸ�Ƭ��̼��Ƶ������������ڼ��н�����̽����ȡ2Ƭ��Ƭ�����˲������У������м���60g �״ף�����ǡ����ȫ��Ӧ�������Ƭ�������ɷֲ�����ᷴӦ������ò����������ʵ�������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ2��ʾ����Ӧ�Ļ�ѧ����ʽ��
��2009?����������ά���������������������Ԫ�أ�С�����õ�ij�ָ�Ƭ�IJ���˵����ͼ1��������֪��ÿ����õĸ�Ƭ��̼��Ƶ������������ڼ��н�����̽����ȡ2Ƭ��Ƭ�����˲������У������м���60g �״ף�����ǡ����ȫ��Ӧ�������Ƭ�������ɷֲ�����ᷴӦ������ò����������ʵ�������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ2��ʾ����Ӧ�Ļ�ѧ����ʽ�� ����ά���������������������Ԫ�أ�ͼΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣�ȡ1Ƭ�ƶ��棬����ʢ��10gϡ������ձ��У�����̼��Ƹ�����ǡ����ȫ��Ӧ�������ɷ��������Ӧ�����ձ�������������Ϊ11.34g������Ӧ����ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2����
����ά���������������������Ԫ�أ�ͼΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣�ȡ1Ƭ�ƶ��棬����ʢ��10gϡ������ձ��У�����̼��Ƹ�����ǡ����ȫ��Ӧ�������ɷ��������Ӧ�����ձ�������������Ϊ11.34g������Ӧ����ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2����