��Ŀ����
����Ŀ��(9������̼����������ָͨ��һ���ķ���������ҵ�����в�����CO2����������д�������á�ʵ�������У�������NaOH��Һ��������CO2����������ͼ��ʾ����������������δ�������
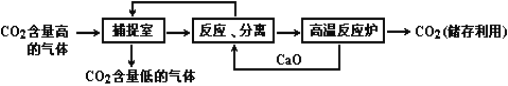
��1�������ڷ�Ӧ�Ļ�ѧ����ʽΪ____________________��
��2������Ӧ���롱�з�Ӧ�Ļ�ѧ����ʽΪ_____________________��__________________ ��
���������õIJ��������� __________________________ ��
��3�������������У�û���漰�Ļ�����Ӧ���͵���_______________��
��4�������йظò����̵�������ȷ����__________________��
A��������CO2���Ʊ�����������Ʒ�����������������ŷ�
B������Ӧ���롱�еķ�ӦҪ���մ�����
C�����������У�ֻ��һ�����ʿ�ѭ������
D���ܺĴ��Ǹò�������һ��ȱ��
���𰸡���1��CO2+2NaOH=Na2CO3+H2O��
��2��CaO+H2O=Ca(OH��2�� Ca(OH��2+Na2CO3=CaCO3��+2NaOH �� ���� ��
��3���û���Ӧ��
��4��A D
��������
���������ʵ�������У�������NaOH��Һ��������CO2�������ڷ�Ӧ�Ļ�ѧ����ʽΪCO2+2NaOH=Na2CO3+H2O������Ӧ���롱�з�Ӧ�Ļ�ѧ����ʽΪCaO+H2O=Ca(OH��2�� Ca(OH��2+Na2CO3=CaCO3��+2NaOH�������˳��������г����к���̼Ԫ�أ����̼Ԫ�صIJ������������õIJ��������ǹ��ˣ������������У�û���漰�Ļ�����Ӧ���͵����û���Ӧ�������̵�������ȷ���в�����CO2���Ʊ�����������Ʒ�����������������ŷţ��ܺĴ��Ǹò�������һ��ȱ�㣻����Ӧ���롱�е�CaO+H2O=Ca(OH��2����ӦҪ�ų������ȣ����������У������ƺͶ�����̼����ѭ�����á�