��Ŀ����
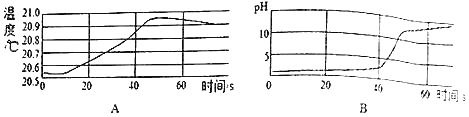
����Ŀ�����۵ĽǶ��˽����ʼ���仯�������ڸ��õ���ʶ���ʵ���ɺͱ仯�ı��ʡ�
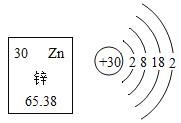
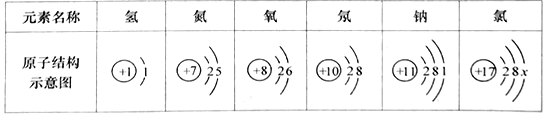
(1)�±�Ϊ����Ԫ�ص�ԭ�ӽṹʾ��ͼ��
��ش��������⣺
�ٱ��о�������ȶ��ṹ��ԭ����___________(��Ԫ�ط���)��
����ԭ�ӵ�����������xΪ___________���ڻ�ѧ��Ӧ����ԭ����___________(��õ�����ʧȥ��)���ӡ�
����ԭ��ʧȥ1�������γ����ӵ����ӷ���Ϊ___________��
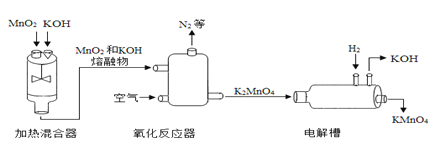
(2)��һ�������£�CO2���軯��(HCN)��Ӧ��������N2����Ӧ����ʾ��ͼ���¡�
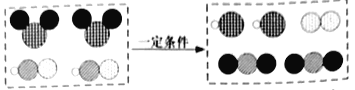
���������е���������ʹ�����ʯ��ˮ����ǣ������ʵĻ�ѧʽΪ___________��
��д����ͼ��ʾ��Ӧ�Ļ�ѧ����ʽ��___________��
���𰸡�Ne 7 �õ� Na+ CO2 2ClO2+2HCN![]() 2HCl+N2+2CO2
2HCl+N2+2CO2
��������
��1��
��ͼ����Ԫ��ԭ�ӽṹʾ��ͼ��������������8Ϊ�ȶ��ṹ������Ne��
����ԭ�ӽṹʾ��ͼ�У��������ͺ����������ȣ�������ԭ�ӵ�����������xΪ17-��2+8��=7������7����������������4���õ����ӣ�����õ���
����ԭ��ʧȥһ�������γɴ�һ����λ����ɵ������ӣ�����Na+��
��2��
����ʹ����ʯ��ˮ�����˵�������˶�����̼���壬����CO2��
�ڸ���ͼʾ��֪�������Ⱥ������ᷴӦ�������ɵ�����������̼���������Ȼ��⣬����ʽ����2ClO2+2HCN![]() 2HCl+N2+2CO2��
2HCl+N2+2CO2��

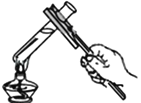
����Ŀ��ij��ȤС��ͬѧ��ʵ�����Ʊ�������������������̽��ʵ�顣
��Ϊ̽�����������������طֽ����ʵ�Ӱ�죬��������¶Ա�ʵ�飺
��� | ʵ��ҩƷ | �ֽ��¶�(��) |
�� | 3.0gKClO3��1.0gMnO2���Ȼ�ϼ��� | 350 |
�� | xgKClO3��1.0gCuO���Ȼ�ϼ��� | 370 |
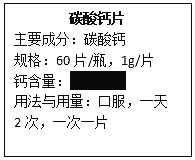
���з�Ӧ�Ļ�ѧ����ʽ��_______��
����x��ֵӦΪ________��
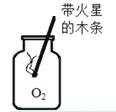
[ʵ�����]ʵ���������ֽ����������У���Ч����õ���_______��
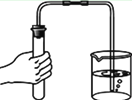
����̽����Ӱ��˫��ˮ�ֽ����ʵ�ij�����أ�ʵ�����ݼ�¼���£�
˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2������ | ��ͬʱ���ڲ���O2��� | |
�� | 50.0g | 1% | 0.1g | 9mL |
�� | 50.0g | 2% | 0.1g | 16mL |
�� | 50.0g | 4% | 0.1g | 31mL |
��ʵ���У�����O2�����װ����______(����)��
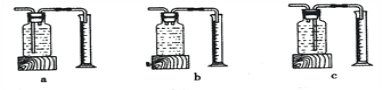
[ʵ�����]����ͬ�����£�_______��˫��ˮ�ֽ��Խ�졣
[��˼]������ͼװ�ý���ʵ�飬ͨ���Ƚ�_____Ҳ�ܴﵽʵ��Ŀ�ġ�