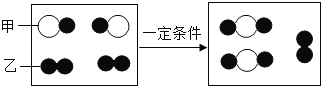
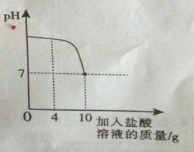
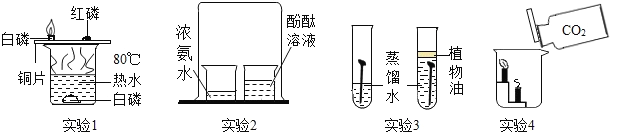
��Ŀ����
����Ŀ��̼��̼�Ļ��������������������Ź㷺��Ӧ�ã�������ش��������⣺
(1)�ڱ����з������̿�ɳ�ȥ�����е���ζ�����������˻���̿��____�ԣ�
(2)��ҵ������������һ����̼��ԭ���������÷�Ӧ�Ļ�ѧ����ʽΪ____
(3)CO2��һ�ֱ������Դ����ѧ�ҷ�����һ������CO2�ķ�������CO2��NH3Ϊԭ�Ϻϳ�����[CO(NH2)2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ____
���𰸡����� 3CO+Fe2O3![]() 2Fe +3CO2 CO2+2NH3==CO(NH2)2+H2O
2Fe +3CO2 CO2+2NH3==CO(NH2)2+H2O
��������
��1���ڱ����з������̿�ɳ�ȥ�����е���ζ�����������˻���̿�������ԣ�
��2����ҵ������������һ����̼��ԭ���������������Ͷ�����̼���÷�Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+3CO![]() 2Fe+3CO2��
2Fe+3CO2��
��3����CO2��NH3Ϊԭ����һ�������ºϳ�����[CO��NH2��2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��CO2+2NH3=CO��NH2��2+H2O��
�ʴ�Ϊ����1����������2��Fe2O3+3CO![]() 2Fe+3CO2����3��CO2+2NH3=CO��NH2��2+H2O��
2Fe+3CO2����3��CO2+2NH3=CO��NH2��2+H2O��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ