��Ŀ����
����Ŀ����ѧ���������ϢϢ��أ������û�ѧ֪ʶ�ش��������⡣
��1��ʪ�·��������±����������ɵÿ죬������Ϊ_____��
��2�������Ҵ������У��Ҵ��������ǣ��û�ѧ����ʽ��ʾ��_____��
��3��ú¯ȼ�չ����з������ȷ�Ӧ���ǣ��û�ѧ����ʽ��ʾ��_____��
��4��ʵ����ʯ��ˮ�ı��泣��һ���Ĥ��ԭ���ǣ���ѧ����ʽ��_____��
��5����ת��������Ч����β���е�CO��NO��NO2��̼�⻯������к�������������ŷš���ת������ͨ�����ò��ȹ��ؽ�������������д��CO��NO�ڴ����������·�����Ӧ����CO2��N2�Ļ�ѧ����ʽ_____��
���𰸡��¶�Խ�ߣ����ӵ��˶�����Խ�죨���¶�Խ��,ˮ������Խ�죩 C2H5OH+3O2 2CO2+3H2O C+CO2
2CO2+3H2O C+CO2 2CO Ca��OH��2+CO2=CaCO3��+H2O 2CO+2NO��2CO2+N2��д����Ҳ���֣�
2CO Ca��OH��2+CO2=CaCO3��+H2O 2CO+2NO��2CO2+N2��д����Ҳ���֣�
��������
��1��ʪ�·��������±����������ɵÿ죬����Ϊ���������¶ȸߣ��¶����ߣ����ӵ��˶����ʼӿ죻
��2�������Ҵ������У��Ҵ�ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��C2H5OH+3O2 2CO2+3H2O��
2CO2+3H2O��
��3��ú¯ȼ�չ����з������ȷ�Ӧ����̼�Ͷ�����̼�ڸ����·�Ӧ����һ����̼����Ӧ�Ļ�ѧ����ʽΪ��C+CO2 2CO��
2CO��
��4��ʵ����ʯ��ˮ�ı��泣��һ���Ĥ��ԭ�������������������̼��Ӧ������̼��ƣ���ѧ����ʽ��ʾΪ��Ca��OH��2+CO2�TCaCO3��+H2O��
��5��CO��NO�ڴ����������·�����Ӧ����CO2��N2����Ӧ�Ļ�ѧ����ʽ�ǣ�2CO+2NO 2CO2+N2��
2CO2+N2��

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�����Ŀ��Ϊ�ⶨ����Na2CO3��ʳ�δ��ȣ�����4�βⶨ��ÿ�ζ�ȡһ����������Ʒ��200g������������Ϊ3.65%�����ᣨ����ΪHCl��������Ʒ���Ƴ���Һ����εμӵ���ȡ�����з�����ӦNa2CO3 +HCl��NaCl+H2O+CO2����δ��ƽ�����������������������õ���Ʒ������ϵ���±���ʾ��
ʵ����� | ��1�� | ��2�� | ��3�� | ��4�� |
����������g�� | 200 | 200 | 200 | 200 |
��Ʒ������g�� | 10.6 | 53 | 265 | 300 |
����������g�� | 0.44 | 2.2 | 4.4 | 4.4 |
����Ʒ��Na2CO3�Ĵ��ȣ�����һλС����д��������̣���__________
����Ŀ��̼��ԭ����ͭ��ʵ����ͼ��ʾ��д���÷�Ӧ�Ļ�ѧ����ʽ________________��
��������⣩̼ȼ��ʱ��������CO2Ҳ��������CO����ô̼������ͭ��Ӧ���ɵ������Ƿ�Ҳ�ж��ֿ���?
���������룩����١�CO ����ڡ�CO2 ����ۡ�________________��
����Ʒ�����ʵ��װ����ͼ��Ũ���������ˮ���ã�����K������ͨ�����ĵ���һ��ʱ�䣬�ر�K����ȼ�ƾ���ƺ;ƾ��ƣ���ַ�Ӧ��Ϩ��ƾ���ƺ;ƾ��ƣ���K����ͨһ��ʱ�䵪����
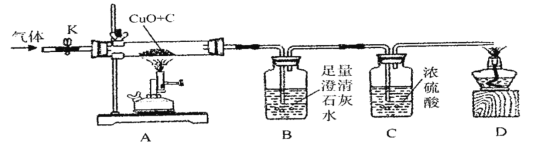
��1��ʵ��ʱA����������___________________��
��2��������ٳ�����B��D����������________________������ţ���
aB�б���ǣ�D���ܵ�ȼ bB�б���ǣ�D�����ܵ�ȼ
cB��������ʵ������D���ܵ�ȼ dB��������ʵ������D�����ܵ�ȼ
�������ôӶ�������Ƕ��ж�
ȡһ����̿�ۺ�4g����ͭ�Ļ�������ʵ�飬���ⶨ�����е��ĸ����ݡ�
��Ӧǰ������ | ��Ӧ������� | |
A��������+���壩 | m1 | m2 |
B+C�����ƿ+���Һ�� | m3 | m4 |
��3��������ڳ������������ϣ�m4һm3��__________��m1��m2����ѡ�<������>����=������
��������˼��ʵ�鿪ʼǰ����ͨ�뵪����Ŀ����_____________________��